- Title
-
Laminin and Matrix metalloproteinase 11 regulate Fibronectin levels in the zebrafish myotendinous junction
- Authors
- Jenkins, M.H., Alrowaished, S.S., Goody, M.F., Crawford, B.D., Henry, C.A.
- Source
- Full text @ Skelet Muscle
|
Fn and laminin are reciprocally expressed at the MTJ adjacent to fast-twitch fibers during muscle morphogenesis. A–C Anterior left, dorsal top, side-mounted, wild-type embryos. Phalloidin staining for actin to visualize the cell morphological changes during muscle morphogenesis. A1 At 16 hpf, muscle precursors are round (arrow in inset) and actin accumulates between somite boundaries (arrowheads). A2 At 24 hpf, muscle cells have elongated (arrow in inset) and actin continues to accumulate at the boundary (arrowheads). A3 At 48 hpf, muscle fibers have undergone further growth and become striated (arrow in inset). MTJs are devoid of actin staining (arrowheads). B1–B3 Focal planes, 3D projections (anterior left, dorsal top, side-mounted), and transverse views ((T) lateral right, dorsal top) of phalloidin (blue) and Fn antibody staining (red). b1 At 18 hpf, Fn accumulates throughout the medial-lateral extent of the MTJ. B2 At 24 hpf, Fn is adjacent to lateral, superficial slow-twitch fibers (arrowheads in T) as well as medial MPs (arrow in focal plane and asterisk in T). B3 At 48 hpf, Fn is downregulated throughout the medial portion of the MTJ except adjacent to MPs (asterisk in T). C1–C3 Focal planes, 3D projections (anterior left, dorsal top, side-mounted), and transverse views ((T) lateral right, dorsal top) of phalloidin (blue) and laminin-111 antibody staining (green). C1 At 18 hpf, laminin-111 begins to polymerize at the MTJ. C2 At 24 hpf, polymerized laminin-111 is adjacent to slow- and fast-twitch muscle fibers (arrowhead in focal plane points to laminin-111 adjacent to fast-twitch muscle fibers). C3 At 48 hpf, laminin-111 remains throughout the medial-lateral extent of the MTJ (arrowhead denotes laminin-111 adjacent to fast-twitch muscle fibers). D, E Models of Fn-laminin dynamics at the MTJ in a medial focal plane (D) and a 3D rendering (E) over developmental time. The presence of slow-twitch fibers (blue) along the midline confirms that focal planes are medial sections. D1 At 18 hpf, a mainly Fn myomatrix segregates somites. D2–D3 In medial focal planes at 24 and 48 hpf, because slow-twitch fibers have migrated laterally, a mainly laminin-111 myomatrix separates myotomes. E1 As slow-twitch fibers migrate laterally, myoblasts elongate and the mainly Fn matrix is replaced by a mainly laminin-111 matrix medial to the location of migrating slow-twitch fibers. E2 After slow-twitch fiber migration is complete, the MTJ is primarily laminin-111 adjacent to fast-twitch fibers while Fn remains adjacent to slow-twitch fibers. Scale bars are 50 μm EXPRESSION / LABELING:
|
|
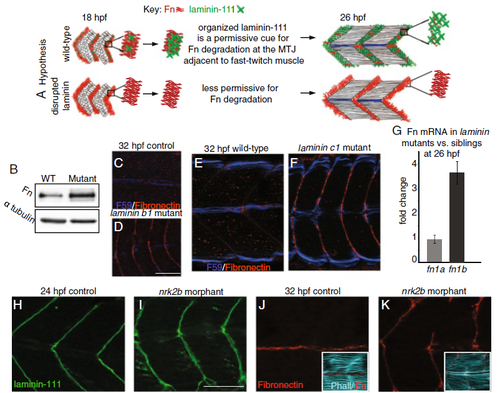
Organized laminin deposition is required for Fn downregulation. a Hypothesis that laminin deposition regulates Fn downregulation. Upper panel: laminin is polymerized at the MTJ and Fn is downregulated. Lower panel: laminin deposition/organization is disrupted and Fn perdures at the MTJ. b Western blot of whole embryo homogenates at 48 hpf using anti-Fn antibody. Laminin gamma1 mutants have approximately 2.5-fold more Fn compared to siblings. Alpha-tubulin was used as a loading control. (C–F; H–K) Anterior left, dorsal top, side-mounted embryos. Fn antibody (red), F59 antibody to label slow-twitch fibers (dark blue), laminin-111 antibody (green), phalloidin staining (light blue). c Medial focal plane of 32 hpf sibling. Note Fn (red) has been downregulated adjacent to fast-twitch fibers. d Medial focal plane of 32 hpf laminin beta1 mutant (n = 16 embryos). Note that Fn persists adjacent to medial, fast-twitch fibers. e Medial focal plane of 32 hpf sibling. Note Fn (red) has been downregulated adjacent to fast-twitch fibers. f Medial focal plane of 32 hpf laminin gamma1 mutant (n = 28 embryos). Note that Fn persists adjacent to medial, fast-twitch fibers in laminin beta1 and gamma1 mutant zebrafish. g Relative mRNA abundance of fn1a and fn1b in laminin gamma1 mutants compared to siblings at 26 hpf (n = 3 biological replicates of at least five embryos each). Fn1a expression is unchanged whereas fn1b expression is upregulated in laminin gamma1 mutants. h Focal plane of laminin-111 antibody staining in a 24-hpf control embryo. i Focal plane of laminin-111 antibody staining in a 24-hpf nrk2b morphant embryo. Note that laminin-111 protein is present, but that the organization of laminin-111 at MTJs is disrupted. j Medial focal plane of Fn antibody staining in a 32-hpf control embryo. k Medial focal plane of Fn antibody staining in a 32-hpf nrk2b morphant embryo (n = 9 embryos). Fn is present at the MTJ adjacent to MPs and fast-twitch fibers in nrk2b morphants. Scale bars are 50 μm |
|
Normal laminin deposition/organization is necessary for concentration of Mmp11 at MTJs. A1–A3 Anterior left, dorsal top, side-mounted, wild-type embryos stained with anti-Mmp11 antibody at 20, 26, or 48 hpf, respectively. Note that Mmp11 increasingly concentrates at MTJs (white arrows) over developmental time. B1, B2 Western blots of whole embryo homogenates at 26 hpf using an anti-Mmp11 antibody. B1 Laminin gamma1 morphants have approximately 8.5-fold less Mmp11 protein compared to controls. B2 Laminin gamma1 mutants show no change (approximately 1.2-fold more) Mmp11 protein compared to siblings. (C–E) Side view, anterior left, dorsal top, 27 hpf embryos stained with phalloidin (light blue), and anti-Mmp11 antibody (purple). C1–C3 Control embryo. Mmp11 localizes to the MTJ (peaks in C3) and is absent from the myotome (valleys in c3). c3 Histogram of relative pixel intensities across the white rectangle in panel c2. d1–d3 nrk2b morphant. Mmp11 is expressed and concentrates at MTJ in nrk2b morphants (white arrow in d2 and peaks in d3); however, Mmp11 localization is disrupted as can be seen by the increased staining within myotomes (higher intensity of valleys in d3 compared to c3) (n = 13 embryos). d3 Histogram of relative pixel intensities across the white rectangle in panel D2. E1–E3 laminin gamma1 mutant. Mmp11 does not localize to MTJs (absence of clear peaks and valleys in E3), except in small patches (white arrowhead in E2) (n = 7 embryos). E3 Histogram of relative pixel intensities across the white rectangle in panel E2. Scale bars are 50 μm EXPRESSION / LABELING:
PHENOTYPE:
|
|
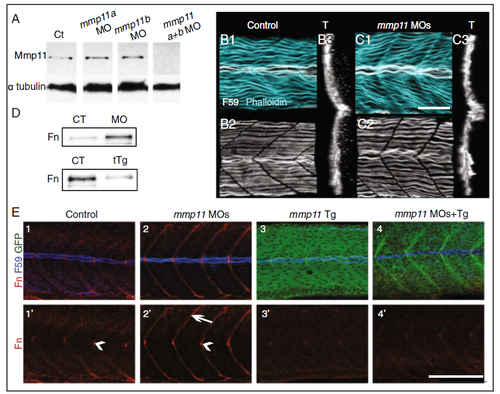
Mmp11 is necessary and sufficient for Fn downregulation. A Western blot of whole embryo homogenates at 26 hpf using Mmp11 antibody. Injection of individual MOs (a or b) alone did not reduce Mmp11 protein levels compared to controls. Co-injection of both MOs (a + b) reduced Mmp11 levels by approximately 14-fold compared to controls. Alpha-tubulin was used as a loading control. B, C Focal planes, 3D projections (anterior left, dorsal top, side-mounted), and transverse views ((T) lateral left, dorsal top) of control embryo (B) and mmp11 morphant (C) at 27 hpf, fast muscle fibers are stained with phalloidin (light blue) and slow-twitch fibers are stained with F59 (white). D Western blot of whole embryo homogenates at 32 hpf (upper panel) or 23 hpf (lower panel) using anti-Fn antibody. Upper panel: Fn protein level is increased by approximately 4.7-fold in mmp11 morphants compared to controls. Lower panel: Fn protein level is decreased by approximately 4.2-fold in embryos overexpressing mmp11-EGFP (tTg) compared to controls. E Medial focal plane, anterior left, dorsal top, side-mounted embryos at 26 hpf stained with Fn antibody (red) and F59 antibody (blue). Overexpression of mmp11-EGFP is green. E1, E1’ Control. (E2–E2’) mmp11 morphant (n > 60 embryos). Note that Fn concentrates adjacent to MPs in both controls and mmp11 morphants (arrowheads in E1’ and E2’) but only concentrates adjacent to fast-twitch muscle fibers in mmp11 morphants (arrow E2’). E3–E3’ mmp11-EGFP transgenic. Note that Fn is downregulated adjacent to MPs in transgenics compared to controls. E4–E4’ mmp11 morphant overexpressing mmp11-EGFP (n > 50 embryos). Mmp11-EGFP overexpression reduced Fn levels throughout the MTJ in mmp11 morphants. Scale bars are 50 μm |
|
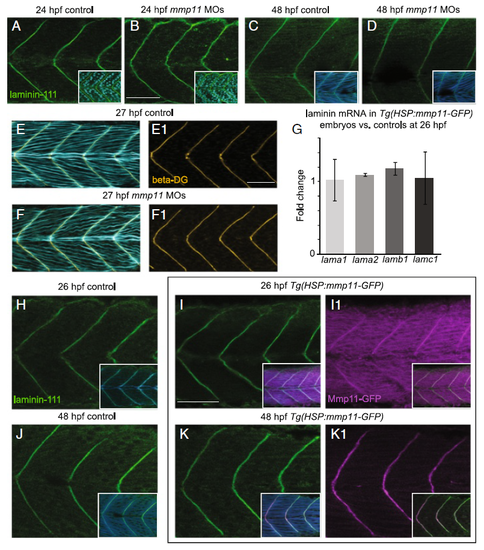
Laminin-111 at MTJs in not affected by Mmp11 knockdown or overexpression. A–F1, H–K1 Anterior left, dorsal top, side-mounted embryos stained for laminin-111 (green), nuclei (light blue), actin (light or dark blue), beta-dystroglycan (orange), or showing Mmp11-GFP (purple). A–B laminin-111 antibody and nuclei staining in 26 hpf embryos. A Control embryo. B mmp11 morphant embryo. C–D laminin-111 antibody and phalloidin staining in 48 hpf embryos. C Control embryo. D mmp11 morphant embryo (n = 8 embryos). Note that laminin-111 appears normal in mmp11 morphants compared to controls at 26 and 48 hpf. E–F1 Beta-dystroglycan antibody and phalloidin staining in 27 hpf embryos. E, E1 Control embryo showing merged panel E and beta-dystroglycan antibody staining alone (E1). F, F1 mmp11 morphant embryo showing merged panel F and beta-dystroglycan antibody staining alone (F1). Normal beta-dystroglycan antibody staining in mmp11 morphants indirectly supports that laminin-111 is unaffected by Mmp11 knockdown. G Relative mRNA abundance of lama1, lama2, lamb1, and lamc1 in mmp11 morphants compared to controls at 26 hpf. The expression of the laminin genes assayed is unchanged by Mmp11-GFP overexpression (n = 2 biological replicates of at least five embryos each). H–K1 laminin-111 antibody and phalloidin staining in heat-shocked AB controls and Tg(HSP:mmp11-GFP) embryos. H Control embryo at 26 hpf showing laminin-111 and laminin-111 merged with phalloidin (inset). I, I1 26 hpf Tg(HSP:mmp11-GFP) embryo showing laminin-111 (I), laminin-111 merged with phalloidin and Mmp11-GFP (inset in I), Mmp11-GFP (I1), and Mmp11-GFP merged with laminin-111 (inset in I1). J Control embryo at 48 hpf showing laminin-111 and laminin-111 merged with phalloidin (inset). K, K1 48 hpf Tg(HSP:mmp11-GFP) embryo showing laminin-111 (K), laminin-111 merged with phalloidin and Mmp11-GFP (inset in K), Mmp11-GFP (K1), and Mmp11-GFP merged with laminin-111 (inset in K1). Note that the timing of Mmp11-GFP MTJ localization recapitulates that of native Mmp11 protein, Mmp11-GFP and laminin-111 co-localize at MTJs, and laminin-111 appears normal in Tg(HSP:mmp11-GFP) embryos (n = 5 embryos). Scale bars are 50 μm |
|
Injection of mmp11 MOs increases Fn at the 48 hpf MTJ in laminin gamma1 mutants and improves slow-twitch fiber morphology. A–D Anterior left, dorsal top, side-mounted, 3D projections of embryos stained for slow-twitch fibers with F59 antibody (blue) and Fn antibody (red). A 32 hpf laminin gamma1 mutant. B 32 hpf laminin gamma1 mutant; mmp11 morphant. C 48 hpf laminin gamma1 mutant. D 48 hpf laminin gamma1 mutant; mmp11 morphant. Note that in 48 hpf laminin gamma1 mutants, Fn is not clearly concentrated at MTJs (C, n = 14 embryos). In contrast, Fn robustly concentrates at MTJs in laminin gamma1 mutants injected with mmp11 morpholinos at 48 hpf (D, n = 8 embryos). E–H Anterior left, dorsal top, side-mounted, 3D projections of embryos stained with anti-F59 antibody. Small inset boxes are brightfield, whole mount, live images. E laminin gamma1 mutant at 24 (inset) or 32 hpf. F laminin gamma1 mutant injected with mmp11 MOs at 24 (inset) or 32 hpf. Note that overall body shape and slow-twitch muscle fibers are similar in both mutants and mutant morphants at 24 and 32 hpf. G laminin gamma1 mutant at 72 hpf. H laminin gamma1 mutant injected with mmp11 MOs at 72 hpf. Slow-twitch muscle fiber detachment can be clearly seen in laminin gamma1 mutant embryos at 72 hpf. However, laminin gamma1 mutants injected with mmp11 MOs have less fiber detachment at this time point EXPRESSION / LABELING:
PHENOTYPE:
|