- Title
-
Innervation regulates synaptic ribbons in lateral line mechanosensory hair cells
- Authors
- Suli, A., Pujol, R., Cunningham, D.E., Hailey, D.W., Prendergast, A., Rubel, E.W., Raible, D.W.
- Source
- Full text @ J. Cell Sci.
|
Ribbons in mechanosensory lateral line hair cells. (A) Transmission electron micrograph (TEM) of a typical spherical ribbon (R) in a mechanosensory hair cell (HC) from a lateral line neuromast in zebrafish. Ribbons are electron-dense structures, anchored to the presynaptic membrane, that tether synaptic vesicles (SV) at synapses with afferent fibers (AF). Vesicles ready to be docked are at the membrane facing the post-synaptic membrane density. (B) At different stages during development, from 2-5days post fertilization (dpf), ribbons at the synapse measure ~300nm in diameter. A one-way ANOVA shows no significant difference (R2=0.2552, P=0.7560); a Tukey′s multiple-comparison test shows a significant difference only between day 3 and 4 ribbons (*P<0.05). Results are mean±s.e.m. |
|
Innervation is required for regulation of ribbon size, number and localization. (A,B) Afferent innervation in neurogenin (neurog1) siblings (sib) (A) and mutants (-/-) (B) as detected by antibody staining for acetylated tubulin. neurog1 mutants (B) lack afferent and efferent (data not shown) innervation of posterior lateral line hair cells but not anterior lateral line hair cells. A total of nine neuromasts (NM) per condition were used for confocal microscopy imaging and ribbon analysis: three posterior lateral line neuromasts (L1, LII.1, LII2) and three anterior lateral line neuromasts (O2, MI1, MI2, IO4 or M2) per larvae were measured in three different larvae. (C-D′) Projections of confocal images of the posterior lateral line neuromasts immunolabeled to show hair cells (green, anti-parvalbumin antibody) and ribbons (magenta, anti-RibeyeB antibody). (E-F′) Lateral views of surface renderings of neuromasts in C-D′ generated by Huygens software. Ribbon size, number and inter-ribbon distance were obtained from the surface renderings of the neuromasts (see Materials and Methods). In neurog1 siblings, ribbons were found at the base of hair cells (E,E′), whereas in neurog1 mutants, ribbons were found both in the cytoplasm and at the base of posterior lateral line hair cells (F,F′). Additionally, in neurog1 mutants, ribbons were fewer in number (G), smaller in average size (H,I) and the distance between ribbons was also increased (J,K). The number of ribbons per cell in anterior lateral line hair cells, which are innervated, did not differ between neurog1 mutants and sibling larvae (L) but ribbon size was smaller in neurog1 mutant hair cells (M). Ribbon size and number were obtained from the surface renderings of the neuromasts (see Materials and Methods). *P=0.09 in H, *P=0.03 in M, ***P<0.0001. Statistical analysis of ribbons (unpaired two tailed t-test) was performed on averages of averages - we calculated the average of ribbons per cell in a given neuromast and then averaged this number across nine total neuromasts (three neuromasts in three different larvae). Posterior lateral line neuromasts in neurog1 mutants had an average of nine hair cells per neuromast, whereas anterior lateral line neuromasts had an average of 11 hair cells per neuromast. Posterior lateral line neuromasts in neurog1 siblings had an average of 12 hair cells per neuromast, whereas anterior lateral line neuromasts had an average of 10 hair cells per neuromast. Results are mean±s.e.m. |
|
Ribbons in neurog1 mutants are cytoplasmic and show an increased pool of synaptic vesicles. (A) TEM transverse section of a posterior lateral line neuromast showing four mature hair cells (HC) surrounded by clearer cytoplasm (arrowheads) of support cells (SC). Even at this low magnification, two ribbons (arrows) are clearly seen. The ribbon in the inset is enlarged in A′ showing more clearly its ectopic position (not anchored at membrane) and a greater amount of surrounding synaptic vesicles (SV) not tethered to the ribbon (R). At the basal pole of the hair cell only the clear cytoplasm of the support cell can be seen. (B) A hair cell from another neuromast shows a double-ribbon, also ectopic, with a profusion of untethered SVs. (C) A ribbon in a wild-type 7dpf hair cell is shown for comparison. A monolayer of SVs surrounds the ribbon. AN, afferent neuron. PHENOTYPE:
|
|
Continuous innervation is required for regulating ribbons in hair cells. Ribbon number and size was assayed in 5dpf larvae with mature neuromast hair cells, whose lateral line fibers were transected on one side of the larva, after the nerves had formed synapses with hair cells. Tg(neuroD:GFP) transgenic animals were used to visualize lateral line afferent fibers in vivo before and after transection. (A) Lateral line afferents in the uncut side of the larva as visualized by acetylated tubulin staining. (B) Lateral line afferents transected (cut) at the level of L1 neuromast in the opposite side of the larva (blue arrow). Cut and uncut larvae were fixed at 24h post transfection and stained for hair cells (green, anti-parvalbumin antibody), ribbons (magenta, anti-RibeyeB antibody) and afferent neurons (cyan, anti-GFP antibody). Projections of confocal microscope images of the uncut (C-C′′) and cut (D,D′) side were acquired at the level of L3, L4 and L5 neuromasts (NM) in the posterior lateral line (A). The absence of afferent fibers in neuromasts of the cut side is seen in D′, which is an overlay of RibeyeB (magenta) and anti-GFP antibody staining (no cyan staining was present), whereas neuromasts in the uncut side continue to be innervated by afferent fibers (C′′). (E-F′) Lateral views of surface renderings of neuromasts in C-D′ generated by Huygens software. In the denervated hair cell, the number of ribbons/cell increased (G), whereas the size of ribbons decreased (H,I). Ribbon size and number were obtained from the surface renderings of the neuromasts (see Materials and Methods). **P=0.0036, ***P=0.0006. Statistical analysis of ribbons (unpaired two tailed t-test) was performed on averages of averages - we calculated the average of ribbons per cell in a given neuromast and then averaged this number across six total neuromasts (three neuromasts in two different larvae). Posterior lateral line neuromasts had an average of 10 hair cells per neuromast in cut larvae and an average of 13 hair cells/neuromast in uncut larvae. Results are mean±s.e.m. |
|
Membrane-adjacent ribbons predominate in mature hair cells. (A-C′′) 5dpf Tg(brn3C:GFP) larvae were treated with 400µM neomycin for 1h to kill all hair cells. At different time points during regeneration larvae were fixed and stained with anti-GFP antibody (green, hair cells) and anti-RibeyeB antibody (magenta, ribbons), and three anterior lateral line neuromasts (NMs) (O2, MI1, MI2, IO4 or M2) were imaged using a confocal microscope. (D) Ribbons seen right next to the basal membrane in the green channel, as visualized in 3D surface renderings (Movies 6 and 7), were counted as basal ribbons, whereas the rest of the ribbons were considered as distal ribbons. At early time points during regeneration both distal ribbons and membrane adjacent ribbons were present in hair cells (12-36 hpt). Distal ribbons eventually disappeared, and by 48h post treatment (hpt) ribbons adjacent to the basolateral membrane predominated. Ribbon numbers were obtained by visually counting ribbons in surface renderings of images of neuromasts (see Materials and Methods). Three neuromasts in three different larvae were imaged and ribbons were counted in a total of 26 hair cells of untreated larvae, 24 hair cells of 12hpt larvae, 51 hair cells of 24hpt larvae, 54 hair cells of 36hpt larvae and 65 hair cells of 48hpt larvae. *P=0.0124, ***P<0.0001 (unpaired two-tailed t-test). Results are mean±s.e.m. |
|
Ultrastructural features of ribbons during hair cell regeneration. (A-I) 5dpf larvae were treated with 400µM neomycin for 1h to kill all hair cells. To assess ribbon morphology during regeneration, larvae were fixed and processed for TEM and anterior lateral line neuromasts were imaged at different times post treatment (hpt). (A-B′) Electron-dense structures indicative of ribbon precursors are found in the cytoplasm of hair cells at early stages (18hpt and 24hpt) during regeneration. (A′,B′) Close up views of boxed regions in A,B. (C-H) Basal ribbons at different stages post treatment. Immature ribbons are seen at early stages such as double ribbons at 12hpt (C) and ‘ectopic’ or ‘floating’ ribbons at 24hpt (E) [the ribbon faces a support cell process (asterisk), which still separates the hair cell from a nearby afferent ending]. The immature ribbon anchored at the membrane at 48hpt (G) faces a growth cone (asterisk), which has the characteristic dense cytoplasm filled with different size vesicles. Mature synapses (D,F,H) were clearly seen from 24hpt to 48hpt. N, nucleus; EN, efferent nerve ending; AN, afferent nerve ending. The images in D-H are shown at the same scale as that in C. |
|
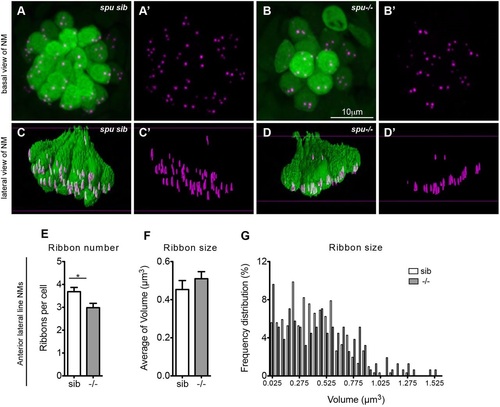
Lack of mechanotransduction does not affect ribbon localization. (A-B′) sputnik (spu) siblings (sib) and mutants, which have a mutation in cadherin 23 and lack tip links in their hair bundles, were fixed at 5dpf and stained for hair cells (green, anti-parvalbumin antibody) and ribbons (magenta, anti-RibeyeB antibody). (A-B′) Projections of confocal images of anterior lateral line neuromasts (NM) (O2, MI1, MI2, IO4 or M2) in siblings and mutants. (C-D′) Lateral view of surface renderings of images in A-B′ generated by Huygens software. spu mutants had one less ribbon/cell (E), but ribbon size (F,G) and their localization (D′) was very similar to wild-type larvae. Ribbon size and number were obtained from the surface renderings of the neuromasts (see Materials and Methods). Statistical analysis of ribbons (unpaired two tailed t-test) was performed on averages of averages: average of ribbons, hair cells or neuromasts in a total of nine neuromasts (three neuromasts in three different larvae). Anterior lateral line neuromasts had an average of six hair cells per neuromast in spu mutant larvae and an average of 10 hair cells per neuromast in spu sibling larvae. *P=0.02 (unpaired two-tailed t-test). The average ribbon size was not statistically different between mutants and their siblings, although the size as assessed by fluorescence microscopy is semi-quantitative. Results are mean±s.e.m. EXPRESSION / LABELING:
|
|
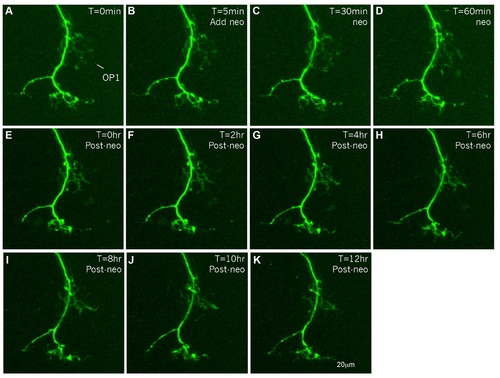
Afferents persist after HC death. Afferent innervation of OP1 anterior lateral line neuromasts; in the anterior lateral line of a 5dpf Tg(neuroD:EGFP) transgenic larva was imaged with a Zeiss spinning disc microscope once every 5 min for a total of 13 hours. After the first image (A) was taken, 400µM neomycin was added to the preparation to kill the HCs (B-D). Following 1 hour of treatment, neomycin was washed out and the afferent innervation was imaged again once every 5 minutes for 12 hours (E-K). Even though HCs die from neomycin treatment (Harris et al., 2003, Ma et al., 2008), afferents still persist and do not significantly retract. At this magnification it is unclear if afferent endings stay within the neuromast or just remain next to it. |

Unillustrated author statements |