- Title
-
INaP selective inhibition reverts precocious inter- and motorneurons hyperexcitability in the Sod1-G93R zebrafish ALS model
- Authors
- Benedetti, L., Ghilardi, A., Rottoli, E., De Maglie, M., Prosperi, L., Perego, C., Baruscotti, M., Bucchi, A., Del Giacco, L., Francolini, M.
- Source
- Full text @ Sci. Rep.
|
Spinal cord and lateral muscle histological analyses. Each adult fish was transversely cut into five segments (S1–S5) using the fins as anatomical references (see Supp. Figure S2). (A) Hematoxylin & eosin-stained histological sections of the S2 segment of the spinal cord (upper panels; scale bar: 50 μm) and white muscle fibres (lower panels; scale bar: 25 μm) in control (Ctrl), wtSod1 and mSod1 zebrafish. Muscular atrophy and edema (*) with infiltrating cells (arrowhead) are visible in mSod1 lateral muscle. (B) The plots show the significant reduction in spinal cord area and the number of motor neurons throughout the spinal cord, and a significant reduction in the calibre of white muscle fibres along the mSod1 fish trunk. Each point in the plots shows the mean value ± SEM of the indicated parameter in each segment of seven animals for each genotype. The measures were statistically analyzed using two-way ANOVA, and corrected by means of Sidak’s multiple comparison test (*P < 0.05; **P < 0.01; ***P < 0.001). PHENOTYPE:
|
|
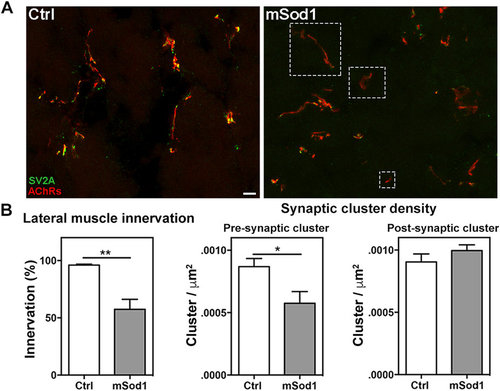
Twelve-month-old mSod1 zebrafish show compromised lateral white muscle innervation. (A) Maximum projections of confocal images of synaptic vesicle protein 2A (SV2A, green) and muscle acetylcholine receptors (AChRs, red) covering the entire thickness (20 μm) of a Ctrl and mSod1 zebrafish lateral muscle cryostat section. In the Ctrl zebrafish, each post-synaptic specialization enriched with acetylcholine receptors faces motor nerve terminals containing vesicle clusters, whereas many of the post-synaptic clusters in the mSod1 section lack an association with motor pre-synaptic terminals (white dashed boxes). Scale bar: 20 μm. (B) The percentage of innervation of post-synaptic specializations is significantly reduced in the mSod1 zebrafish (57.48 ± 8.69% vs 95.94 ± 0.84%), and three-dimensional co-localization analysis of z-stacks covering the entire thickness of the sections revealed a significant reduction in pre-synaptic cluster density (5.76 ± 0.09 vs 8.69 ± 0.06 × 10−4 clusters/μm2) but not in post-synaptic cluster density (9.96 ± 0.05 vs 9.04 ± 0.06 × 10−4 clusters/μm2). The columns in each graph indicate the mean value ± SEM of the indicated parameter in five Ctrl and six mSod1 zebrafish. The measures were statistically analyzed using an unpaired Student t-test (*P < 0.05; **P < 0.01). |
|
Sod1 overexpression causes motor nerve alterations at 24 hpf. (A) Confocal fluorescence maximum projection images showing SV2A signals (green) in the 12–16th somite region of the entire trunk of control (Ctrl), wtSod1 and mSod1 zebrafish embryos at 24 hpf (the same analysis was made in the 17–21st somite region with comparable results, data not shown). As synaptic vesicles travel the entire axonal length, it is possible to observe the length of embryonal motor axons and motor nerve branches. Scale bar: 25 μm. (B) Both wtSod1 (78.9 ± 2.8 μm) and mSod1 embryos (73.1 ± 3.5 μm) showed significantly shorter motor axons than the Ctrl (95.6 ± 2.4 μm) and a significant decrease in unbranched axonal length (55.0 ± 3.5 and 38.0 ± 4.3 μm vs 73.0 ± 2.8 μm), but only the mSod1 embryos showed a significant increase in the number of motor nerve branches: 3.8 ± 0.4 vs 1.9 ± 0.2 (Ctrl) and 2.4 ± 0.3 (wtSod1). The columns indicate the mean value ± SEM of the indicated parameter in at least five motor nerves of each of 25 Ctrl, 17 wtSod1, and 21 mSod1 embryos. The measures were statistically analyzed using one-way ANOVA or the Kruskal-Wallis test, respectively corrected by means of Tukey’s or Dunn’s multiple comparison test (*P < 0.05; ***P < 0.001). |
|
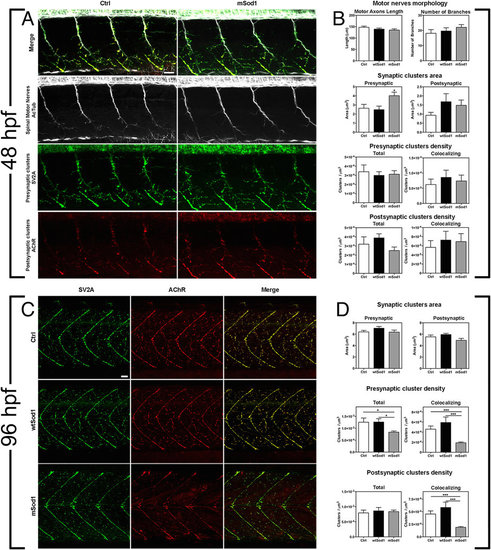
mSod1 embryos and larvae show defects in the clustering of synaptic vesicles and the maturation of neuromuscular junctions. (A) Confocal analysis of the 9–13th somite trunk region at 48 hpf. Scale bar: 25 μm. (B) There were no differences among the control (Ctrl), wtSod1 and mSod1 embryos and larvae in terms of motor axon length (respectively 147.2 ± 5.2 μm, 139.3 ± 4.6 μm, and 135.1 ± 5.5 μm), the number of branches (18.1 ± 2.5, 19.7 ± 2.1, and 22.1 ± 1.8), or unbranched axon length (28.9 ± 4.8 μm, 6.6 ± 2.1 μm, and 7.9 ± 2.2 μm) (data not shown). The measurements were made in at least four motor nerves of a minimum of 10 embryos for each genotype. Three-dimensional (3D) object-based co-localization analysis (numerical data given in Table 1) did not reveal any differences in the densities of total pre-synaptic or pre-synaptic co-localizing clusters, or in the densities of total post-synaptic or post-synaptic co-localizing clusters, but there was a significant increase in the area of pre-synaptic (but not post-synaptic) clusters in the mSod1 embryos. (C) Confocal analysis of the 10–15th somite trunk region at 96 hpf. Scale bar: 25 μm. (D) Although the mean area of the pre- and post-synaptic clusters in the different genotypes remained unchanged, 3D object-based co-localisation analysis showed a significant reduction in the density of presynaptic (but not post-synaptic) SV2A clusters in mSod1 NMJs, and a significant reduction in co-localizing pre- and post-synaptic clusters in mSod1 larvae (numerical data given in Table 1). |
|
mSod1 larvae show a significant reduction in the calibre of muscle fibres with a preserved sarcomere ultrastructure. (A) Maximum projection of myosin second harmonic generation (SHG) signal images in the 12–15th somite region of the trunk of mSod1 larvae. Scale bar: 25 μm. (B) Myosin SHG signal images of Ctrl, wtSod1 and mSod1 muscle fibres showing the endogenous myosin signal to quantify muscle fibre calibre (scale bar: 20 μm), which was significantly reduced in the mSod1 than in the control (Ctrl) or wtSod1 larvae (5.13 ± 0.16 μm vs 6.28 ± 0.26 μm and 5.82 ± 0.30 μm). The columns indicate the mean value ± SEM in 17 Ctrl, 15 wtSod1 and 18 mSod1 larvae. The data were statistically analyzed using one-way ANOVA and Tukey’s multiple comparison tests (**P < 0.01). (C) Detail of myosin SHG signal showing sarcomere organization in Ctrl, wtSod1 and mSod1 muscle fibres. Scale bar: 5 μm. No difference in the length of the sarcomeres in the Ctrl, wtSod1 and mSod1 fibres has been detected. (D) Electron micrographs showing the preserved sarcomere ultrastructure in Ctrl, wtSod1 and mSod1 larvae at 96 hpf. Lack of variation in sarcomeres length was confirmed by measuring 100 sarcomeres in two fish per genotype (respectively 1.89 ± 0.03 μm, 1.85 ± 0.03 μm, and 1.90 ± 0.04 μm). The measures were statistically analyzed using one-way ANOVA, corrected by means of Tukey’s post-test. PHENOTYPE:
|
|
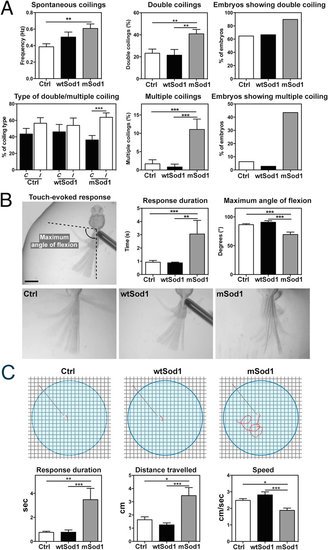
mSod1 embryos and larvae show increased locomotor activity. (A) In comparison with controls (Ctrl), mSod1 embryos showed a significant increase in the frequency of spontaneous tail coiling behavior at 20 hpf. The mSod1 embryos showed a significantly higher percentage of both double and multiple coiling in comparison with the Ctrl and wtSod1 embryos. The Ctrl and wtSod1 embryos showed the same percentage of contralateral (C) and ipsilateral (I) bends of the entire body, whereas the mSod1 embryos showed a significant higher percentage of I coilings (numerical data given in Table 2). (B) The maximum angle of tail flexion (scale bar: 500 μm) and the duration of touch-evoked responses at 48 hpf. Touch-evoked tail coiling responses lasted significantly longer in the mSod1 embryos, and the maximum angle of tail flexion was significantly less (numerical data given in Table 3). (C) Schematic representation of the experimental set-up used to test touch-evoked swimming responses in larvae at 96 hpf, showing a representative response in red. The mSod1 larvae showed significantly longer-lasting evoked swimming responses and travelled significantly further; as these responses consisted of repeated consecutive burst swimming events, the average speed was significantly lower (numerical data given in Table 3). PHENOTYPE:
|
|
Riluzole treatment reverts the motor phenotype and normalizes motor axon length in mSod1 embryos. PHENOTYPE:
|
|
Riluzole treatment reduces spontaneous high-frequency depolarizations in the spinal motor neurons of mSod1 embryos. PHENOTYPE:
|
|
Riluzole treatment reduces spontaneous high-frequency depolarizations in the spinal interneurons of mSod1 embryos. PHENOTYPE:
|
|
The macroscopic anatomy and body weight of adult zebrafish are not affected by Sod1 over-expression. (A) Typical traits measured to compare the macroscopic anatomy of the transgenic and Ctrl zebrafish: standard length (SL), the length between the operculum and the caudal peduncle (LOCP), and height at the anterior of the anal fin (HAA). Scale bar: 0.5 cm. (B) Each fish was transversely cut into five segments (S1-S5) using the fins as anatomical references. Scale bar: 0.5 cm. (C) Representative pictures of 12-month-old zebrafish: an adult of the AB line (Ctrl), and zebrafish expressing wtSod1 or mSod1. Scale bar: 0.5 cm. (D) The histograms show SL, HAA, LOCP measured in seven Ctrl, six wtSod1, and seven mSod1 fish, and the body weight recorded in 15 Ctrl, 14 wtSod1, and 14 mSod1 fish. The columns indicate the mean values ± SEM of the indicated parameter, and the results were statistically analyzed using one-way analysis of variance (ANOVA) and the Kruskal-Wallis test, corrected by means of Dunn’s multiple comparison test. There were no significant differences among the three genotypes. |
|
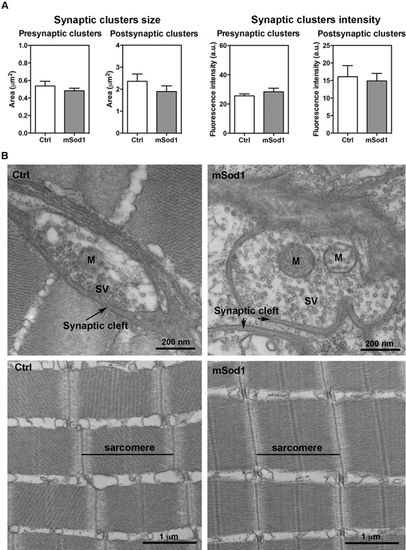
Twelve-month-old zebrafish neuromuscular junction (NMJ) and lateral white muscle structure. (A) The 3D co-localization analyses of z-stacks covering the entire thickness of the lateral muscle sections of the mSod1 and control (Ctrl) fish did not reveal any significant differences in pre-synaptic (0.48 ± 0.03 μm2 vs 0.54 ± 0.05 μm2) or post-synaptic cluster size (1.89 ± 0.26 μm2 vs 2.36 ± 0.33 μm2), or in the fluorescence intensity of the pre-synaptic (28.34 ± 2.42 a.u. vs 25.56 ± 1.39 a.u.) or post-synaptic clusters (14.88 ± 2.16 a.u. vs 16.09 ± 3.16 a.u.). The columns indicate the mean values ± SEM of the indicated parameter in five Ctrl and six mSod1 adult zebrafish. The measures were statistically analysed using an unpaired Student t-test. (B) Representative electron microscopy images of the NMJ (upper panel) and muscle ultrastructure (lower panel) in Ctrl and mSod1 adults. M: mitochondria; SV: synaptic vesicles. |
|
Adult transgenic Sod1 zebrafish show spinal cord reactive astrogliosis but not microgliosis, but only mSod1 fish have activated inflammatory cells in their lateral white muscles. (A) Reactive astrogliosis and microgliosis were analysed by immunofluorescence in histological sections of 12-month-old zebrafish spinal cord with GFAP (a marker of astrocytes) and Aif1 (a marker of activated microglia in spinal cord and activated macrophages and neutrophils in peripheral tissues), and evaluating the ratio between the mean fluorescence intensity (FI) of both markers and the corresponding spinal cord area (μm2) of segments S2-S5. Each point in the graphs represents the mean values ± SEM of the indicated parameter in each segment of seven Ctrl, six wtSod1 and seven mSod1 zebrafish. There was a significant increase in the fluorescence intensity of GFAP in both transgenic spinal cords, but there were no differences in the Aif1 signals. Scale bar: 25 μm. (B) Confocal images of Aif1-stained S4 lateral white muscle. Scale bar: 25 μm. The atrophic muscle fibres of mSod1 zebrafish are surrounded by areas enriched in activated macrophages and neutrophils: the graphs show a significant increase in the number of Aif1-positive puncta and the percentage area of lateral white muscle covered by Aif1-positive puncta in all of the examined segments. Each point indicates the mean values ± SEM of the indicated parameter in three adult zebrafish of each genotype. The measures were statistically analysed using two-way ANOVA, corrected by means of Sidak’s post-test. *P<0.05; ***P<0.001; ****P<0.0001 |
|
Morphological and ultrastructural changes in developing zebrafish locomotor network. Control (Ctrl) embryos 24 hpf (upper panels) show short motor axons protruding from the spinal cord entirely filled with synaptic vesicles (stained with anti-SV2A antibodies - green). This outgrowth has a rostral to caudal developmental pattern, and only the more rostral motor nerves show branches. Acetylated tubulin (grey) stains a few spinal interneuronal axonal projections. At this developmental stage, no clusters of acetylcholine receptors (AChRs - red) are visible on muscle fibre precursors. Scale bar: 20 µm. The ultrastructural analysis confirmed that the axonal projections are filled with synaptic vesicles (sv), with immature boutons (B) facing muscle fibres precursors with glycogen-filled cytoplasm (square box) and a poorly organized contractile apparatus. Scale bar: 500 nm. By 48 hpf (middle panels), the motor nerves present a well-organized microtubule network along their entire length, deeply penetrating the trunk along myosepta, and begin innervating the branched muscle fibre precursors. The axons are no longer entirely filled with synaptic vesicles, which are well organized in small clusters at the tips of the axonal branches, and the muscle fibres begin to show visible clusters of AChRs (red). Electron micrographs show pre-synaptic terminals filled with vesicles (sv), mainly located at the periphery of myotomes, and muscle fibres with a well-organized contractile apparatus (S). By 96 hpf (lower panels), the larvae show well-developed, heavily branched motor nerves innervating muscle fibres. Synaptic vesicles are distributed in small clusters at the tips of the axonal terminals, which now face the AChR clusters on muscle fibers (their superimposition generates a yellow signal in the merged image). Ultrastructural analysis reveals small pre-synaptic boutons (B) deeply penetrating into the myotome and innervating well-developed muscle fibres. The confocal images are maximum projections of z-stacks covering half of the trunk. Symbols: AZ: active zone; M: pre-synaptic mitochondria; m: muscle mitochondria; e: endosome. |
|
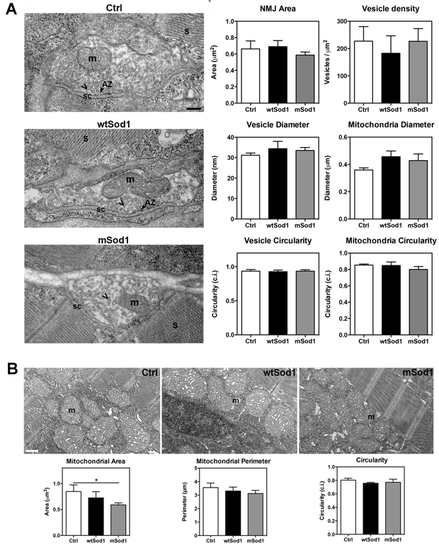
The ultrastructure of mSod1 neuromuscular junctions (NMJs) is preserved at 96 hpf. (A) Electron micrographs showing the neuromuscular junction ultrastructure of 96 hpf control (Ctrl), wtSod1 and mSod1 larvae. The pre-synaptic boutons contained many vesicles (chevron) widely distributed in the pre-synaptic terminals without any particular polarisation, some which are fusing in the active zone (AZ). The pre-synaptic terminals also included mitochondria (m); they are separated from the muscle fibres by a well-defined synaptic cleft (sc). The junctions did not show any clear post-synaptic specialisations. Well-organized sarcomeres (S) were visible in muscle fibres. Scale bar: 200 nm. The morphometric analyses did not reveal any differences in neuromuscular junction area (Ctrl: 0.66 ± 0.10 µm2; wtSod1: 0.69 ± 0.07 µm2; mSod1: 0.59 ± 0.04 µm2), or synaptic vesicle density (Ctrl: 227.10 ± 53.33 n/µm2; wtSod1: 182.50 ± 64.10 n/µm2; mSod1: 226.70 ± 46.38 n/µm2), diameter (Ctrl: 31.20 ± 1.07 nm; wtSod1: 34.42 ± 3.60 nm; mSod1: 33.55 ± 1.44 nm), circularity (Ctrl: 0.94 ± 0.02 circularity index [c.i]; wtSod1: 0.92 ± 0.02 c.i.; mSod1: 0.93 ± 0.02 c.i.), area (Ctrl: 588.80 ± 36.45 nm2; wtSod1: 695.10 ± 128.90 nm2; mSod1: 677.40 ± 52.55 nm2; not shown) or perimeter (87.08 ± 3.32 nm in Ctrl, 94.83 ± 10.28 nm in wtSod1, 93.46 ± 4.31 nm in mSod1; not shown) or in mitochondria number (Ctrl: 0.95 ± 0.15; wtSod1: 0.90 ± 0.20; mSod1: 0.80 ± 0.10), diameter (Ctrl: 0.36 ± 0.02 µm; wtSod1: 0.46 ± 0.04 µm; mSod1: 0.43 ± 0.05 µm), circularity (Ctrl: 0.85 ± 0.01 c.i.; wtSod1: 0.85 ± 0.04 c.i.; mSod1: 0.80 ± 0.04 c.i.), area (Ctrl: 0.08 ± 0.01 µm2; wtSod1: 0.12 ± 0.03 µm2; mSod1: 0.08 ± 0.01 µm2; not shown) or perimeter (Ctrl: 1.07 ± 0.06 µm; wtSod1: 1.28 ± 0.14 µm; mSod1: 1.11 ± 0.05 µm; not shown) in the pre-synaptic terminals. The columns represent the mean values ± SEM of the indicated parameter in 30 neuromuscular junctions of three fish of each genotype. The measures were statistically analysed using the Kruskal-Wallis test, corrected by means of Dunn’s multiple comparison procedure. (B) Electron microscopy images showing muscle mitochondria (m) morphology in Ctrl, wtSod1 and mSod1 larvae. Scale bar: 500 nm. Muscle mitochondria area was significantly smaller in the mSod1 larvae (Ctrl: 0.89 ± 0.09 μm2; wtSod1: 0.82 ± 0.12 μm2; mSod1: 0.54 ± 0.04 μm2), but there was no significant difference in mitochondria perimeter (Ctrl: 3.66 ± 0.23 μm; wtSod1: 3.44 ± 0.25 μm; mSod1: 2.93 ± 0.19 μm) or circularity (Ctrl: 0.81 ± 0.02 c.i.; wtSod1: 0.79 ± 0.03 c.i.; mSod1: 0.79 ± 0.03 c.i). The measurements refer to one hundred mitochondria in six Ctrl, four wtSod1 and six mSod1 larvae, and were statistically analysed using one-way ANOVA, corrected by means of Tukey’s multiple comparison tests (*P<0.05). |
|
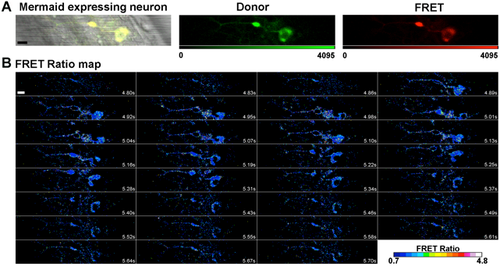
Mosaic expression of HuC:Mermaid vector in a 20 hpf zebrafish spinal motor neuron, and a FRET ratio map of a spontaneous depolarisation event. (A) In order to verify whether the aberrant motor phenotype observed in 20 hpf mSod1 embryos was associated with alterations in the spontaneous depolarisation of spinal neurons, one-cell-stage embryos were micro-injected with the FRET-based voltage biosensor Mermaid ORF under the control of the pan-neuronal promoter HuC. The bright field image merged with the fluorescence signal shows efficient biosensor expression in a spinal motor neuron. The detected donor and FRET channel are also shown. Scale bar: 10 µm. (B) The time points on the FRET ratio map of the same motor neuron during a spontaneous depolarisation event shows that the FRET ratio increases with membrane potential. Scale bar: 10 µm. |