- Title
-
ECM couples the convergence movements of mesoderm and neural plate during the early stages of neurulation
- Authors
- Araya, C., Carmona-Fontaine, C., Clarke, J.D.
- Source
- Full text @ Dev. Dyn.
|
Depletion of LamC1 and Fn1 lead to severe disruption of neural tube. A-A′′: Expression of laminin (Lam) from neural plate to neural tube stages seen in transverse sections. B-B′′: Expression of Fibronectin (Fn) from neural plate to neural tube stages seen in transverse sections. C: Wild-type embryos showing normal expression of the apical marker zonula occludens (ZO-1, red) by 24 hpf. D,E: Knockdown of either Fn1 or LamC1 results in only minor disruption to ventricle morphology as revealed by ZO-1 expression. F: Knockdown of both Fn1 and LamC1 results in more severe disruption to ventricle and neural tube morphology. The normally midline expression domain of ZO1 becomes fragmented and ectopic (yellow arrowheads). Mes, mesoderm; np, neural plate; IV, fourth ventricles in the hindbrain; OV, the otic vesicle; hpf, hours post fertilization. |
|
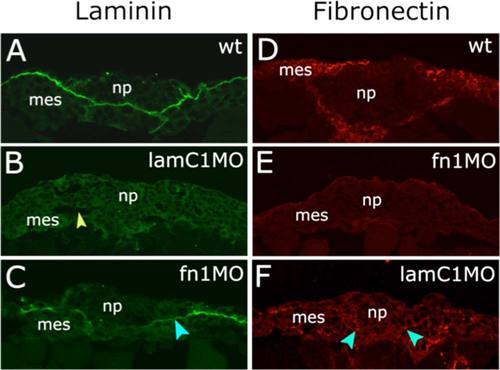
lamininC1 and fibronectin1 morpholino efficiencies at neural plate stages. A-F: Transverse sections of zebrafish embryos at 11.5 hpf. Laminin immunoreactivity is shown in green in A, B, and C. Fibronectin immunoreactivity is shown in red in D, E, and F. A: Lam reactivity at 11.5 hpf between neural plate and mesoderm. B: Reduction of Lam by lamC1 MO (arrowhead indicate tissue gaps between neural plate and mesoderm). C: After fn1MO, Lam reactivity shows slightly reduced levels (arrowhead). D: Fn reactivity at 12 hpf. E: fn1MO strongly reduces Fn expression. F: After lamC1MO, Fn reactivity shows reduced levels (arrowheads). np, neural plate; mes, mesoderm; hpf, hours post fertilization. |
|
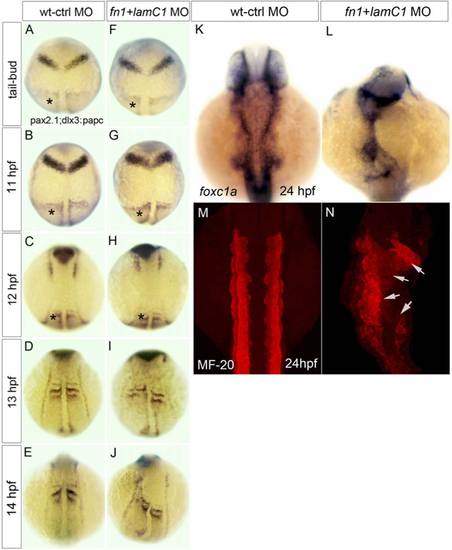
ECM is required for neural and mesoderm morphogenesis. A-J: Analysis of the neural markers pax2.1 and dlx3 and the mesoderm marker papc (asterisked) show that disrupted tissue organization only arises between 12 and 13 hpf in Fn1 and LamC1 deficient embryos. K-N: At 24hpf, the mesoderm markers foxc1a and MF-20 show disrupted distribution of mesoderm in Fn1 and LamC1 deficient embryos. Arrows in N, indicates tissue disruption. hpf, indicates hours post fertilization. |
|
ECM is required to maintain close tissue apposition of neural tissue and mesoderm. A-C: Selected frames from a time-lapse sequence showing close apposition of mesoderm and neural tissue throughout neurulation in wild-type embryo. There is no space apparent between mesoderm and neural tissue. By 20 hpf (540 min) the neural tube and mesoderm remains closely apposed. D-F: Time-lapse sequence from an Fn1/LamC1 deficient embryo showing the presence of gaps between the neural plate and mesoderm from early time point of neurulation (arrowheads). By 20 hpf, tissue gaps are still present (arrowheads) and neural tube architecture becomes impaired and no clear midline is seen. In A-F, the enveloping layer (evl) has been pseudocolored in blue, neural plate (np) has been pseudocolored in yellow, and the mesoderm (mes) has been pseudocolored in red. G: Analysis of cell division orientation and location shows the normal midline location of divisions is lost and stereotyped mediolateral orientation of divisions is disrupted in Fn1/LamC1 deficient embryos. hpf, hours post fertilization; min, minutes and asterisks mean embryo dorsal midline. |
|
Extracellular matrix (ECM) is required to couple movements of neural plate and mesoderm. A: First frame of time-lapse from wild-type (wt) embryo. Image is in transverse plane and includes left-hand side and midline of neural plate (orientation shown in E). Evl, indicates selected cells in enveloping layer (blue dots), neural plate (yellow dots), and mesoderm (red dots). Arrow indicates midline. Cell nuclei are labeled with H2A-GFP. B: Projection of frames 1 to 10 of time-lapse movie from wt embryo, showing the tracks of the selected cells in the EVL, neural plate and mesoderm indicated in A. C: First frame of time-lapse from embryo depleted of laminin and fibronectin by morpholino injection. Image is in transverse plane and includes left-hand side and midline of neural plate (orientation shown in E). Evl, indicates selected cells in enveloping layer (blue dots), neural plate (yellow dots), and mesoderm (red dots). Arrow indicates midline. Cell nuclei are labeled with H2A-GFP. D: Projection of frames 1 to 10 of time-lapse movie from laminin and fibronectin depleted embryo, showing the tracks of the EVL, neural plate and mesoderm cells indicated in C. E: Diagrams indicating orientation and position of time-lapse movies used for images in A to D and analyses in F to I. Blue layer is enveloping layer (EVL), yellow layer is neural plate (NP) and red layer is mesoderm (MES). F: Trajectories of neural (yellow) and mesodermal (red) nuclei in laminin and fibronectin depleted embryos. G-I: Analyses in cell directionality (G), persistence (H), and angular speed (I), reveals uncoupled behavior of neural plate and mesoderm cells in Fn1/LamC1 morpholno embryos. |
|
Neural plate and mesoderm remain coupled in Has2 deficient embryos. A: Frame 1 from a time-lapse sequence of a has2 deficient embryo. A neural plate (np) nucleus is marked with a yellow dot, a mesodermal (mes) nucleus with a red dot, and enveloping layer (evl) nucleus with blue dot. Arrow indicates tissue movements. Midline of neural plate marked with an arrowhead. Orientation and position as for Figure 5E. B: Frames 1 to 10 of time-lapse superimposed to reveal parallel tracks of neural and mesodermal nuclei. C: Trajectories of neural plate (yellow) and mesoderm (red) nuclei on Has2 depleted embryo. D-F: Analyses in cell directionality (D), persistence (E), and angular speed (F), reveals movements of neural plate and mesoderm cells remain coupled in Has2 depleted embryos. |