- Title
-
Six3 regulates optic nerve development via multiple mechanisms
- Authors
- Samuel, A., Rubinstein, A.M., Azar, T.T., Ben-Moshe Livne, Z., Kim, S.H., Inbal, A.
- Source
- Full text @ Sci. Rep.
|
Optic disc coloboma and optic nerve malformations in six3a;six3b double mutant embryos. (A) Schematic presentation of WT Six3a and Six3aL183S proteins. Amino acid numbers are depicted and location of L183S in the homeodomain is marked with an arrow. HD, homeodomain. (B) Chromatograms showing the sequence difference between WT (left) and mutant (right) six3a. (C-F) Lateral views (C,D) and ventral views (E,F) of 3 dpf live normal (C,E) and Six3-deficient (D,F) embryos. Arrows in (C,D) point at lenses, arrowheads in F point at the medial, incomplete RPE. (G-J) Low (G,H) and higher (I,J) magnifications of histological transverse sections through forebrains of normal (G,I) and Six3-deficient (H,J) 5 dpf embryos. “R” in (H) marks ectopic retinal tissue of one eye. Arrowhead and arrows in I point at the optic nerve inside and outside the eye, respectively, which cannot be clearly seen in the double mutant; asterisks mark location of the optic chiasm; bracket in J marks an apparently defasciculated optic nerve. (C,D,G-J) dorsal is up, (C-F) anterior is to the left. Scale bars are 50 µm. PHENOTYPE:
|
|
Optic nerve misrouting in Six3-deficient embryos. (A-H) Maximum projection confocal images of labelled RGC axons in WT (A,C,E,G) and Six3-deficient (B,D,F,H) embryos. (A,B) zn-5 immunohistochemistry at 40 hpf. Asterisk in A marks the optic chiasm and in (B) where the optic chiasm should be. Arrows in B point at RGC axons. Rectangles in (A,B) mark the GCL of one eye. (C,D) Ac-T immunohistochemistry at 48 hpf. Asterisks in (C,D) mark location of the optic chiasm. Arrow in (D) points at a region where RGC axons are misrouted and fail to exit the eye. Insets in (C,D) show higher magnification of the regions at which arrowheads point. (E,F) DiO anterograde labelling from a single eye at 5 dpf. Arrow in F points at axons reaching the forebrain. (G,H) DiO (green) and DiI (red) labelling of VT and ND RGCs, respectively, at 3 dpf. t, tectum. In (H) arrow points at VT axons remaining ipsilaterally and arrowhead points at VT axons misrouted to the forebrain. (A-D) are ventral views and E-H are dorsal views. Anterior is up. Scale bars are 50 µm. |
|
Eyes and optic stalks of Six3-deficient embryos are abnormally patterned. (A-L) WISH of 30 hpf normal (A,C,E,G,I,K) and Six3-deficient (B,D,F,H,J,L) embryos. (A,B) vax1 expression. Brackets marks POAs, arrows point at OSs. (C-F) vax2 expression in the POA, OS and ventral retina. Brackets marks POAs, arrows point at OSs. t, telencephalon. (G,H) pax2a expression in the optic fissure (arrowheads in G,H) and OS (arrow in G, missing in H). (I,J) tbx5 expression in dorsal retina. White lines mark the width of the region where high tbx5 levels are observed. (K,L) raldh2 expression in the dorsal retina. Arrows mark limits of high raldh2 expression. (A-D) are ventral views, anterior is up. E-L are lateral views, anterior to the left, dorsal up. Scale bars are 50 µm. |
|
Abnormal optic stalk development in Six3-deficient embryos. (A-D) WISH for pax2a at 18 hpf (A,B) and 24 hpf (C,D) in WT (A,C) and Six3-deficient embryos (B,D). Arrows in (A-D) point at the OSs. (E-H) Confocal images of OS in live WT (E,G) and Six3-deficient (F,H) embryos at 23-24 hpf (E,F) and 29-30 hpf (G,H). Outlines of all cells are highlighted by expression of membrane-tethered GFP. White lines demarcate OS width at the interface with the retina. (I-L) Confocal images of rx3:Kaede live embryos in WT (I,K) and Six3-deficient (J,L) backgrounds. The OS was photoconverted in these embryos (red). (I,J) Images were captured immediately after photoconversion at 24 hpf. (K,L) Images of the same embryos as in I and J, respectively, at 48 hpf. Arrows point at the periphery of the optic nerve, where only in the WT embryo (K) photoconverted glial cells are present. (M,N) WISH for cxcl12a in WT (M) and Six3-deficient (N) embryos at 30 hpf. Arrows in M point at OS expression of cxcl12a. b, brain; e, eye; t, telencephalon. (A-D) dorsal views anterior up; (E-J) frontolateral views, dorsal up; (K,L) ventral views anterior up; (M,N) frontal views, dorsal up. Scale bars are 50 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Abnormal midline signalling in Six3-deficient embryos. (A-H) Expression of signalling molecules in anterior diencephalic midline of normal (A,C,E,G) and Six3-deficient (B,D,F,H) 32 hpf embryos. (A-F) frontal views, (A′-F′) the same embryos as in (A-F) respectively, lateral views. (A,A′,B,B′) slit1a expression. Asterisk in (B) marks an apparently single commissure. (C,C′,D,D′) slit2 expression. Asterisk in (D) marks what appears as a single commissure. (E,E′,F,F′) slit3 expression. Asterisk in F marks the reduced POA. Brackets in all lateral views mark the POA flanked by AC and POC. (G,H) sema3d expression. Arrows point at the POA. AC, anterior commissure; POA, preoptic area; POC, post-optic commissure; t, telencephalon. (G,H) are dorsal views, anterior down. Anterior is to the left in lateral views. Scale bars are 50 µm. |
|
The POA is severely reduced in Six3-deficient embryos. (A,B) ntn1a expression at 32 hpf in normal (A) and Six3-deficient (B) embryos. Insets are dorsal views of the same embryos. Asterisk and arrowhead (inset) in A mark the ntn1a-negative POA. (C,D) Ac-T immunohistochemistry at 28 hpf in normal (C) and Six3-deficient (D) embryos. Asterisk in (C) marks the POA. (E,F) isl1 expression at 24 hpf in normal (E) and Six3-deficient (F) embryos. Brackets mark the dorsoventral dimension ventral to the anteriormost telencephalon. AC, anterior commissure; POA, preoptic area; POC, post-optic commissure; t, telencephalon; h, hypothalamus. (A,B,E,F) are lateral views, anterior to the left; (C,D) are frontal views. Scale bars are 50 µm. |
|
Delayed differentiation of RGCs in Six3-deficient retinas. (A-L) Eyes in whole embryos (A-F) or dissected eyes (G-L). (A,B) zn-5 immunohistochemistry of eyes in normal (A) and Six3-deficient embryos (B). Arrow in (B) points at the anterior-ventral region lacking differentiated RGCs. (C-F) WISH for cxcr4b at 30 hpf (C,D) and 48 hpf (E,F) in normal (C,E) and Six3-deficient (D,F) retinas. (G-J) WISH for isl1 at 35 hpf (G,H) and 48 hpf (I,J) in normal (G,I) and Six3-deficient (H,J) retinas. Asterisk in H marks remnants of tissues outside the eye, which was dissected. (K-L) WISH for robo2 at 48 hpf in normal (K) and Six3-deficient (L) retinas. Arrows in (K,L) point at RGCs that express robo2. All panels are lateral views, anterior to the left. Scale bars are 50 µm. |
|
Results of normal six3a0 and six3avu129 misexpression. Live embryos at 26 hpf after injection of synthetic RNA encoding normal or mutant Six3aL183S. Four phenotypic groups were scored: normal looking, mild: slightly dorsalized, medium: moderately dorsalized and severe: strongly dorsalized. The percentage of embryos from each group after injection of different doses of normal or mutant six3a RNA is shown in the graph, with phenotypes color-coded. The number (n) of embryos in each group and injection doses are depicted on top and bottom of bars, respectively. |
|
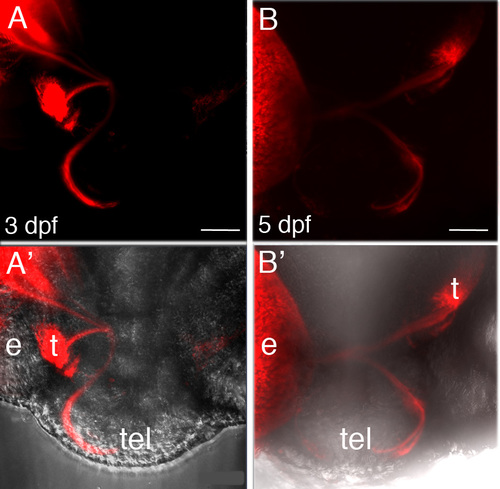
RGC axon misrouting in Six3-deficient embryos. (A, B) Two examples from 3 and 5 dpf Six3-deficient embryos, of RGC axon misrouting with axons projecting from one eye both to tectum and telencephalon. (A′, B′) are the same images as A and B, respectively, with overlay on bright field image to help visualize anatomy. Eyes were labelled with DiI. e, eye; t, tectum; tel, telencephalon. |
|
Early neural plate patterning appears normal in six3a;six3b double mutants. (A-D) Wild-type embryos (A,C) and embryos from crosses between six3a;six3b double heterozygous parents (n>100 for each labelling) (B,D) were labelled by in situ hybridization for eye field marker rx3 (A,B) and neural plate marker fezf2 (C,D) at early segmentation. All embryos in clutches from six3a;six3b double heterozygous parent appeared similar and comparable to wild type. (C,D) Arrows and arrowheads point at presumptive telencephalon and ventral diencephalon, respectively. |