- Title
-
Deltex1 is inhibited by the Notch-Hairy/E(Spl) signaling pathway and induces neuronal and glial differentiation
- Authors
- Cheng, Y.C., Huang, Y.C., Yeh, T.H., Shih, H.Y., Lin, C.Y., Lin, S.J., Chiu, C.C., Huang, C.W., Jiang, Y.J.
- Source
- Full text @ Neural Dev.
|
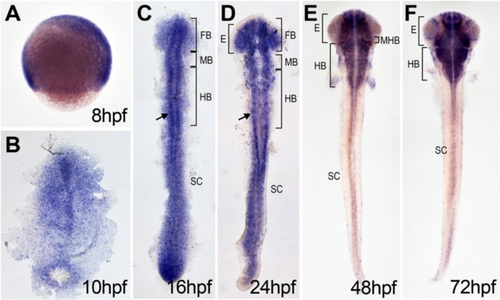
dtx1 expression in developing zebrafish. dtx1 expression was detected using in situ hybridization in the developing nervous system during zebrafish embryogenesis. The embryo stages are shown in the bottom right corner of each panel. a Lateral view with anterior to the right. b–e Dorsal view with anterior to the top. b–f dtx1 expression occurred first in the developing nervous system during the bud stage (b) and remained until the final stage that was analyzed (e) Arrows in c and d indicate dtx1 expressing cells flanking the midline. E, eye; FB, forebrain; HB, hindbrain; MB, midbrain; MHB, midbrain-hindbrain boundary; SC, spinal cord EXPRESSION / LABELING:
|
|
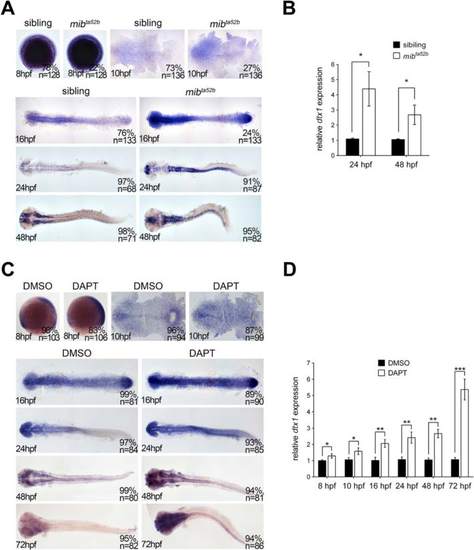
dtx1 expression is upregulated in Notch-deficient embryos. a The expression of dtx1 was analyzed in mib ta52b homozygous mutants and wild-type siblings through in situ hybridization. The stages are shown in the bottom left corner. Embryos were produced by crossing the parents with heterozygous mutant genotype. Embryos at 8 hpf, 10 hpf, and 16 hpf stages were mixed genotypes containing heterozygous mib ta52b mutation, homozygous mib ta52b mutation, and wild-type mib, and they were grouped according to the substantial difference in dtx1 expression. The results indicate that approximately 75 % of wild-type and heterozygous mib ta52b siblings had unaltered dtx1 expression, whereas approximately 25 % of homozygous mib ta52b mutants had increased dtx1 expression. Embryos at 24 hpf and 48 hpf were grouped according the morphological defects that only appeared in mib ta52b homozygous mutants. c. DAPT treatment was performed at different time points, and the embryos were harvested at different stages as indicated; the results show that DAPT treatment caused upregulation of dtx1 expression. b and d The results of in situ hybridization in a and c were quantitatively confirmed using qPCR analysis, respectively. *, P < 0.05 EXPRESSION / LABELING:
PHENOTYPE:
|
|
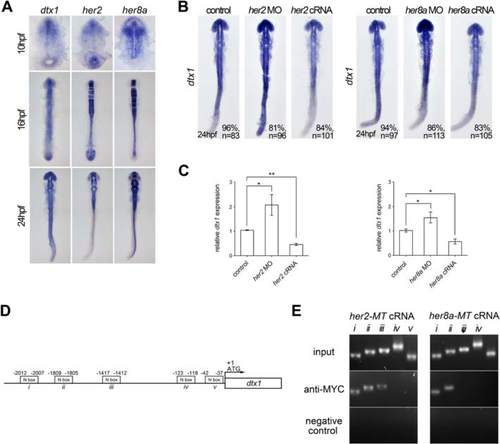
Her2 and Her8a inhibits dtx1 transcription through direct binding to the promoter region. a Comparison of the expression of dtx1 with her2 and her8a showing partially overlapped expression in the developing nervous system. b In situ hybridization result showing that her2 or her8a cRNA inhibited dtx1 expression. c qPCR results showing that her2 or her8a cRNA injection downregulated dtx1expression. *, P < 0.05; **, P < 0.01. d A schematic representation of the promoter region of dtx1, and fragments containing potential N-boxes are named and indicated. These fragments were selected for PCR amplification. e Chromatin immunoprecipitation (ChiP)–PCR analysis was performed on the bud stage embryos. Many fragments (i, ii, and iii) that were amplified by PCR suggested the direct binding of Her2 and Her8a to these regions |
|
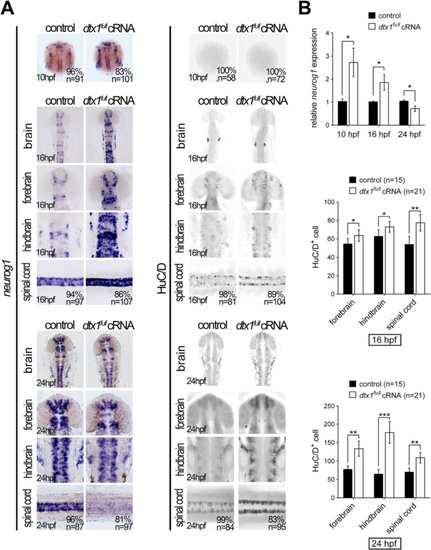
Dtx1 induces the premature differentiation of neuronal precursors. Embryos were examined through in situ hybridization by using neurogenin1 and immunohistochemistry by using an anti-HuC/D antibody. a Compared with the controls, neurogenin1 expression level was increased at the bud stage but downregulated in dtx1 full -injected embryos. By contrast, a significant upregulation of HuC/D signals was detected in dtx1 full cRNA-injected embryos. b The results in a were confirmed using qPCR and cell count. Quantitative data are presented as mean ± standard deviation normalized to the number of controls. *, P < 0.05; **, P < 0.01 |
|
Disruption of Dtx1 expression by using dtx1 ΔIII or dtx1 morpholino reduces neuronal differentiation. a The injection of dtx1 ΔIII or dtx1 morpholino caused an identical phenotype, which downregulated neurog1 (a) and HuC/D expression (b) The phenotypes caused by the morpholino injection could be rescued by a concomitant injection of dtx1 full cRNA. The embryo stages are shown in the bottom left corner of each panel. c qPCR analysis confirmed the results obtained through in situ hybridization in a d Counting the HuC/D-positive cells confirmed the results obtained in b *, P < 0.05; **, P < 0.01; ***, P < 0.001 |
|
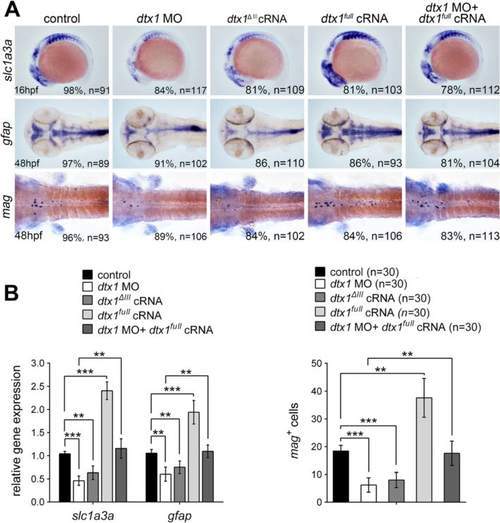
Dtx1 induces the expression of markers for glial precursors and mature glial cells. a Expression of slc1a3a, mag, and gfap was induced in embryos that overexpressed dtx1 full . An injection with dtx1 ΔIII or dtx1 morpholino downregulated slc1a3a, mag, and gfap expression, and this effect could be rescued by a concomitant injection of dtx1 full cRNA. The embryo stages are shown in the bottom left corner of each panel. b qPCR analysis and cell count confirmed the results obtained through in situ hybridization shown in A. *, P < 0.05; **, P < 0.01; ***, P < 0.001 EXPRESSION / LABELING:
PHENOTYPE:
|
|
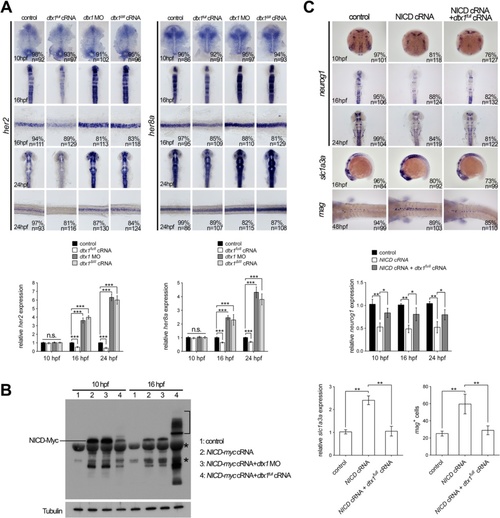
Dtx1 regulates Notch signaling in a temporal manner. a In situ hybridization and qPCR results show that expression of her2 and her8a was not affected by the alteration of dtx1 expression at 10 hpf. In contrast, the expression of her2 and her8a was downregulated by dtx1 full cRNA and upregulated by dtx1 ΔIII or dtx1 morpholino after 16 hpf. The embryo stages are shown in the bottom left corner of each panel. b Injection of NICD inhibits neurog1 expression and induces slc1a3a and mag expression. This effect of NICD could be abrogated by co-injection with dtx1 full cRNA at all stage examined. The result of in situ hybridization was confirmed by qPCR quantification showing in a and b c Western blot analysis with anti-Myc antibody revealed that injection of dtx1 full cRNA caused ubiquitination of NICD-myc, indicated by multiple bands in samples at 16 hpf (bracket). Asterisks indicating non-specific bands PHENOTYPE:
|
|
Her2 and Her8a specifically bind to the N-box in dtx1 promoter. a. Green fluorescence indicates EGFP expression driven by the fragment containing N-boxes, which is overlapped with HuC/D-positive neurons (red). b. The intensity of EGFP expression in HuC/D-positive cells, quantified by ImageJ, showing co-injection of N-box:EGFP with full-length her2 or her8a cRNA downregulated the expression of EGFP, whereas co-injection of a her2 or her8a construct lacking the transcription activating WRPW domain (her2 ΔWRPW or her8a ΔWRPW , respectively) with N-box:EGFP does not downregulate EGFP expression. |
|
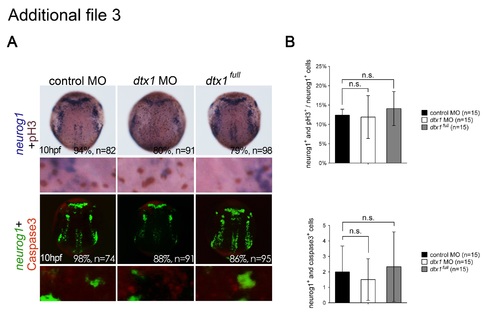
Disruption of Dtx1 expression had no effects on neuronal proliferation and apoptosis. a. Proliferating neurons were double-labeled with phospho-histone H3 antibody (brown) and neurogenin1 riboprobes (purple). Apoptotic neuronal precursor cells were labeled for neurogenin1 (fluorescent green) and activated caspase-3 antibody (fluorescent red). b. Proliferating neuronal cells were quantified by counting the proportions of phospho-histone H3- and neurogenin1-positive cells, whereas apoptotic neural progenitor cells were quantified by counting the proportions of activated caspase-3- and neurogenin1-positive cells, revealing no marked differences between the embryos injected with dtx1 full , dtx1 morpholinos, and the controls. n.s., nonsignificant. |
|
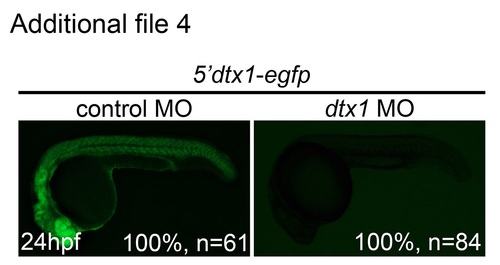
dtx1 Morpholino specifically and effectively downregulated Dtx1 function. Embryos injected with the construct containing dtx1 morpholino-binding sequence fused upstream of egfp (5′dtx1:egfp) alone or in combination with a control morpholino or a dtx1 morpholino. Compared with controls, eGFP expression in dtx1 morphants was downregulated. |
|
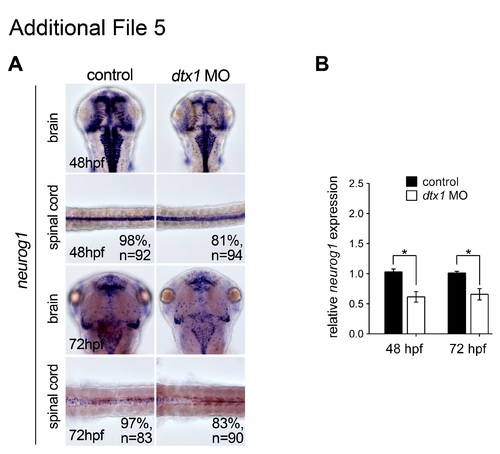
Injection of dtx1 morpholino downregulates neurog1 after 24 hpf. neurog1 expression level was downregulated from 48 hpf to 72 hpf in dtx1 morpholino injected embryos analyzed by in situ hybridization (a) and qPCR (b). *, P < 0.05. |
|
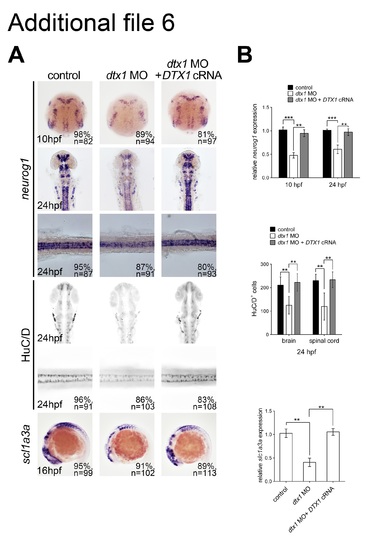
The phenotypes caused by morpholino injection could be rescued by human DTX1 cRNA. Concomitant injection of human DTX1 cRNA with dtx1 MO1 rescued the neuronal and glial phenotypes caused by Dtx1 knockdown analyzed by in situ hybridization (a) and qPCR (b). (TIF 3052 kb) |
|
Phenotypes of Dtx1 morphants were not caused by nonspecific p53 activation. Embryos coinjected with dtx1 MO and tp53 MO were compared with Dtx1 morphants, and we observed no detectable deviation from all markers tested. Compared with the controls, the injection of tp53 MO alone did not show any significant alterations, as shown by in situ hybridization (a), immunohistochemistry (b), qPCR (c), and cell count (D).*, P < 0.05; **, P < 0.01; n.s., nonsignificant. |