- Title
-
N-Ethylmaleimide-Sensitive Factor b (nsfb) Is Required for Normal Pigmentation of the Zebrafish Retinal Pigment Epithelium
- Authors
- Hanovice, N.J., Daly, C.M., Gross, J.M.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
au18 and au13 mutants are oculocutaneous albinos. (A, B) Lateral and dorsal whole embryo images of phenotypically wild-type sibling and mild and severe au18 mutants at 48hpf and 72hpf. (C, D) Images of phenotypically wild-type sibling and mild and severe au13 mutants at 48hpf and 72hpf. In both lines, severe mutants display large regions devoid of pigment in the RPE. PHENOTYPE:
|
|
The RPE is disrupted in au18 mutants. Images of sibling and mutant embryos show that the RPE of au18 embryos is hypopigmented at 48 (A, C, E), 58 (G, I, K), and 72 hpf (M, O, Q). Transverse histologic sections of sibling and mutant embryos at 48 (B, D, F) 58 (H, J, L), and 78 hpf (N, P, R) reveal that au18 eye is microphthalmic, but overall retina structure appears to be normal. Areas devoid of pigment are marked by white arrowheads. Scale bar: 20 µm. (B2, D2, F2, H2, J2, L2, N2, P2, R2) Magnified images of RPE show that RPE proximal to the optic nerve was consistently depigmented in au18 and confirm that au18 RPE appears to have fewer melanosomes than wild-type siblings. The posterior lens also appears to be cataractous in severe au18 mutants (F, L, R). Scale bar: 10 µm. PHENOTYPE:
|
|
au13 and au18 phenotypes are likely caused by mutations in nsfb. (A) Schematic of the nsfb protein. There are three major domains: the Nsf-N, ATPase domain 1 (D1), and ATPase domain 2 (D2). The mutation occurs at nucleotide 893 (C893T), and the dotted red line indicates the location of the premature stop codon at amino acid 297 (S297X) in au18 mutants. This mutation is predicted to truncate the protein by 60%. (B) Graph illustrating the percentage of phenotypically mutant embryos in each clutch following injection of vehicle, nsfbWT, and nsfbau18 mRNA. Both au13 and au18 mutant phenotypes are rescued by injection of nsfbWT, whereas injection of nsfbau18 mRNA failed to rescue either the au13 or au18 mutant phenotype. (C) Representative images of WT, au18+/-, and au18-/- embryos following nsfbWT injection. (D) Quantitative PCR quantification of the relative fold change of nsfb in nsfbau13 and nsfbau18 embryos at 48 hpf. Transcript levels were normalized to act2b. Error bars indicate SEM of five technical replicates for each experiment. n = 3 biological replicates. |
|
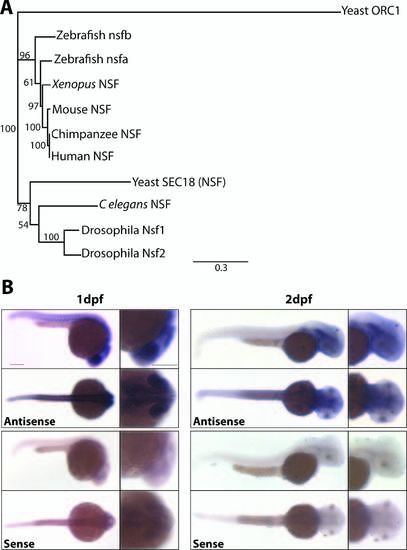
nsfb is a paralog of human NSF and is ubiquitously expressed during early embryo development. (A) Phylogenetic analysis of the relationship between nsf in vertebrates. This analysis was constructed using Geneious Tree Builder based on an alignment of nsf amino acid sequences from human, chimpanzee, mouse, Xenopus, zebrafish, Drosophila, C. elegans, and yeast, using yeast Origin recognition complex subunit 1 (ORC1) as an outgroup. Numbers at nodes represent bootstrap values after 1000 replicates. (B) In situ hybridization reveals that nsfb expression is widespread at 24 hours after fertilization, with higher levels of staining in the somites and nervous system. At 48 hpf, nsfb remains widely expressed in the head, with high levels of expression in the otic vesicle and pectoral fins, but is less apparent in the somites and posterior trunk. Lateral and dorsal views of embryos stained for antisense probe are on top, and sense controls on bottom. Scale bar: 200 µm. |
|
Melanosome formation and maturation is disrupted in nsfbau18 mutants. (A) Cartoon schematic showing the dorsal, central, and ventral regions of the RPE that were analyzed. RPE between 20° and 40° clockwise from an imaginary line (dotted line) drawn between the center of the lens and the center of the optic nerve was defined as central, whereas RPE 80° to 100° was defined as dorsal. Finally, RPE contained within a box 30° to 50° counterclockwise was defined as ventral. (B) Representative TEM images of central RPE for sibling, mild, and severe nsfbau18 RPE at 48, 58, and 72 hours after fertilization. Images oriented distal (choroid) up, proximal (retina) down. Scale bar: 1 µm. (C) Quantification of the average number of melanosomes per square micrometer of RPE indicates that nsfbau18 mutants contained significantly fewer melanosomes than sibling at all time points, and severe nsfbau18 mutants contained significantly fewer melanosomes than mild nsfbau18 mutants at 48 and 72 hpf. (D) The graph depicting the average percentage of melanosomes containing pigment reveals that nsfbau18 melanosomes were significantly less mature than sibling at all time points and that severe mutants were less mature than mild. (E) Graph depicting the average perimeter of melanosomes. Although the average perimeter of melanosomes in sibling and mild nsfbau18 mutants was not significantly different, severe nsfbau18 melanosomes were significantly smaller than sibling. Furthermore, although melanosomal perimeter increased in sibling and mild RPE, severely affected mutant melanosomes decreased over time. (Mann-Whitney U test, *P < 0.01, **P < 0.001, ***P < 0.0001). PHENOTYPE:
|
|
au18 is histologically indistinguishable from sibling at 24hpf. Transverse histological sections of sibling and mutant embryos at 1dpf reveal that homozygous nsfbau18 mutant retinae are histologically normal when compared to WT siblings (A,B). Scale bar = 20µm. Additionally, there does not appear to be any reduction in pigmentation in the RPE (A′,B′). Scale bar = 10µm. |