- Title
-
Spatially differentiated expression of quadruplicated green-sensitive RH2 opsin genes in zebrafish is determined by proximal regulatory regions and gene order to the locus control region
- Authors
- Tsujimura, T., Masuda, R., Ashino, R., Kawamura, S.
- Source
- Full text @ BMC Genet.
|
The RH2-LCR is an evolutionarily conserved enhancer in teleosts. a Sequence comparison of the RH2 locus between zebrafish and medaka (M) is shown using mVISTA program. The baseline corresponds to the zebrafish RH2 region. An enlarged illustration around the RH2-LCR is also shown. Black and gray bars under the chart are the exons of the RH2 genes and the other genes, respectively. The red bar indicates the RH2-LCR. The sequence homology is indicated to the right of the chart. b Sequence comparisons of the RH2 locus of medaka with zebrafish (Z) and Tetraodon (T) are shown as in (a). Homology regions colored with gray correspond to coding regions of genes and those colored with pink correspond to conserved non-coding sequences. Note that Tetraodon has higher homology at the RH2-LCR than zebrafish, reflecting their closer relation with medaka. c Construction of the medaka RH2-A/GFP-BAC clones (upper panel) and expression levels at 5 dpf of the GFP reporter in zebrafish injected with the BAC clones above (lower panel). The histogram shows the percentage of eyes graded into four levels (+++, ++, + and -) according to the number of GFP-expressing cells in the retina. The names to the left of the histogram indicate the constructs injected. The numbers to the right of the histogram show the total number of eyes examined. d, e Whole mount retinas of 7-dpf zebrafish embryos injected with the RH2-A/GFP-BAC (d) and with mixture of the medaka RH2-LCR fragment and the GFP reporter under the 3-kb upstream region of RH2-A. GFP fluorescent signals appear as green (left) and immunostaining signals of SDCs by the anti-RH2 antibody appear as magenta (middle). Overlap of the two signals appears as white. The right panels are the overlays of the left and middle panels. Scale bars = 10 µm |
|
GFP expression patterns specified by the RH2 upstream sequences. a The promoter of keratin 8 attached with the RH2-LCR was linked to a GFP reporter (top left). The GFP was mostly expressed in the SDCs with some ectopic expression as indicated by a white arrowhead (bottom left). The transverse sections of the retinas of the adult transgenic zebrafish showed GFP expression in the entire region from the dorsal to the ventral retina (right). Scale bars = 10 µm (bottom left), 100 µm (right). b Schematic representation of the RH2 upstream constructs with the RH2-LCR. c Images of the transverse sections of the retinas of the adult transgenic zebrafish possessing the respective constructs. The dorsal side is at the top and the ventral side is at the bottom. d Vertical sections of the photoreceptor layer in the same adult retinas as in (c). Immunostaining signals of SDCs by the anti-RH2 antibody appear as magenta, GFP fluorescent signals appear as green, and overlap of the two signals appears as white. Note the weak ectopic expression by the keratin 8 and RH2-3 upstream construct in non-SDC photoreceptor cells as indicated by white arrowheads (a, d). Scale bars = 100 µm (c) and 10 µm (d) |
|
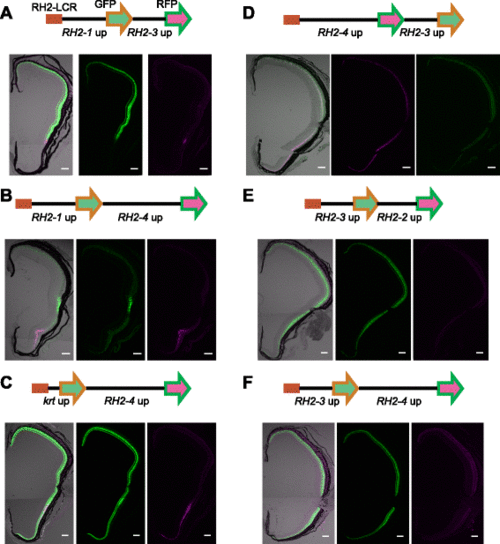
Presence of a competitive promoter modulates reporter expression by the RH2-LCR and RH2 upstream elements. a-f Schematic representations of constructs with the RH2-LCR and double promoter-reporter sets are depicted at the top. RH2-LCR is represented as a red rectangle. The GFP and RFP reporters are represented as green and magenta arrows, respectively. The upstream sequences used to drive the reporters are indicated below. The lower panels are transverse sections of retinas from the adult transgenic fish with the respective constructs. The middle and right panels are fluorescence from the first and second reporters, respectively. The left is the overlay of the middle and right panels with DIC images of the same retina. The GFP signals appear as green and the RFP signals appear as magenta. Note that the signal in the right panel of (f) is autofluorescence from the retinal pigment epithelium as evident in the overlaid image. The dorsal side is at the top, and the ventral side is at the bottom. Scale bars = 100 µm |
|
Translocation of the RH2-LCR revealed gene-order dependent competition for the RH2 regulation. a Translocation of the RH2-LCR. The RH2-LCR was removed from the original position and then re-inserted into the immediate downstream of RH2-3 in the RH2-PAC clones [18]. b Transverse sections of the adult transgenic zebrafish retinas of the PAC constructs where RH2-2 (left), RH2-3 (middle) and RH2-4 (right) are replaced with the GFP reporter respectively. The GFP signals appear as green. Note that in the left panel the green signal is saturated and only the autofluorescence from the retina is captured. The dorsal side is at the top, and the ventral side is at the bottom. Scale bars = 100 µm |
|
Simultaneous recapitulation of the RH2-1 and RH2-2 expression by the GFP and RFP reporters. (A) GFP and RFP reporters were inserted in the place of RH2-1 and RH2-2, respectively, in the RH2-PAC clone. (B-E) The reporter expression was analyzed in the transgenic fish carrying the RH2-PAC construct of (A). The GFP signals appear as green and the RFP signals appears as magenta. The overlay of the two signals appears as white. The right panels are the overlays of the left and the middle panels. (B) A whole mount retina of a 7-dpf fish. Images are superimposed view of stacked sectional images serially obtained using confocal laser scanning microscopy. The dorsal side is at the top. (C) Expanded views of the central area of a whole mount retina shown in (B). (D) A whole mount retina of an 11-dpf fish as shown in (B). (E) A transverse section of the adult retina. The dorsal side is at the top and the ventral side is at the bottom. Scale bars = 50 µm (B, D), 10 µm (C) and 100 µm (E). |
|
Simultaneous recapitulation of the RH2-3 and RH2-4 expression by the GFP and RFP reporters. (A) GFP and RFP reporters were inserted in the place of RH2-3 and RH2-4, respectively, in the RH2-PAC clone. (B-D) The images of the retina of the transgenic fish carrying the RH2-PAC construct of (A). The GFP signals appear as green and the RFP signals appear as magenta. The overlay of the two signals appears as white. The right panels are the overlays of the left and the middle panels. (B) A whole mount retina of a 10-dpf fish. Images are superimposed view of stacked sectional images serially obtained using confocal laser scanning microscopy. The dorsal side is at the top. (C) A transverse section of the adult retina. The dorsal side is at the top and the ventral side is at the bottom. (D) Expanded views of (C). There are photoreceptor cells expressing both GFP and RFP. Scale bars = 50 µm (B), 100 µm (C) and 20 µm (D). |
|
The GFP and RFP expression in the transgenic fish of the double promoterreporter constructs with the RH2-LCR. (A-B) Schematic representations of constructs with the RH2-LCR and double promoter-reporter pairs are depicted at the top. The RH2-LCR is represented as a red rectangle. The GFP and RFP reporters are depicted as green and magenta arrows, respectively. The upstream sequences used to drive the reporters are indicated below. The lower panels are transverse sections of retinas from the adult transgenic fish carrying the respective constructs. The middle panel is fluorescence from the first reporter and the right one is that from the second reporter. The left is the overlay of the middle and right panels with DIC images of the same retina. The GFP signals appear as green and the RFP signals appear as magenta. The dorsal side is at the top, and the ventral side is at the bottom. Scale bars = 100 µm. |