- Title
-
Nanobody-targeted E3-ubiquitin ligase complex degrades nuclear proteins
- Authors
- Ju Shin, Y., Kyun Park, S., Jung Jung, Y., Na Kim, Y., Sung Kim, K., Kyu Park, O., Kwon, S.H., Ho Jeon, S., Trinh, l.e. .A., Fraser, S.E., Kee, Y., Joon Hwang, B.
- Source
- Full text @ Sci. Rep.
|
FACS and microscopic analyses of H2B-GFP expression after transient transfection of synthetic E3 ligase candidates. (a) FACS analysis of H2B-GFP/293TetOn stable cell line transiently transfected with various synthetic E3 ligase candidates. After transfection, Myc10-TM (transmembrane form of Myc10 epitope) and each candidate ligase were simultaneously expressed from a bi-directional tetracycline response element (TRE) promoter following doxycycline treatment (Supplementary Fig. 2). vhhGFP4 is a nanobody against GFP. NSnoFbox was derived from NSlimb by deleting its F-box domain necessary for binding to the adaptor protein in the Skp1-Cullin1-F-box E3 ubiquitin ligase complex9. ‘–’ represents expression of Myc10-TM without any candidate E3 ligase. Only vhhGFP4-SPOP E3 ligase greatly depleted H2B-GFP in the cells expressing Myc10-TM. (b) Microscopic analysis of H2B-GFP/U2OS stable cell line transiently transfected with the ligase candidates. Doxycycline (1 µg/ml) was administered for 24 hours to promote expression of TagRFP and each ligase candidate. Each panel shows bright field, TagRFP (red), H2B-GFP (green), and merged images of both TagRFP and H2B-GFP. Only vhhGFP4-SPOP or vhhGFP4-SPOPΔNLS has no yellow nuclear signal in merged images because of H2B-GFP depletion in cells expressing TagRFP. |
|
Selective depletion of nuclear GFP-fusion proteins by Ab-SPOP. 293TetOn cells expressing Cdk4-GFP, Elf3-GFP, cMyc-GFP, or Pin1-GFP were transfected with vector expressing Ab-SPOPΔNLS or Abmutation-SPOPΔNLS from a bi-directional TRE promoter. ′–′ indicates untransfected cells. Expression of TagRFP and depletion of GFP were measured 8 hours after adding doxycycline (1 µg/ml) to media. Selective depletion of GFP in the nucleus, but not cytoplasm, was only observed in cells transfected with Ab-SPOP. |
|
Time course of nuclear cMyc-GFP depletion by Ab-SPOP. 293TetOn cells stably expressing cMyc-GFP from a CMV promoter and RFP/Ab-SPOP from a bi-directional TRE promoter were imaged under epifluorescence at different time points (0, 4, 5, 6, 7, 8, 12 h) after doxycycline (1 µg/ml) administration. Overlay merges images of c-Myc-GFP and RFP. |
|
The proteasome inhibitor MG-132 blocks H2B-GFP degradation by Ab-SPOP. 293TetOn cells expressing H2B-GFP from a CMV promoter and FLAG-Ab-SPOP/RFP from a bi-directional TRE promoter were examined under epifluorescence 0 h (a) or 24 h (b) after doxycycline (1 µg/ml) and MG-132 (5 µg/ml) treatments. (C) H2B-GFP and FLAG-Ab-SPOP proteins levels were also investigated by immunoblotting with anti-GFP, anti-FLAG, and anti-actin antibodies. |
|
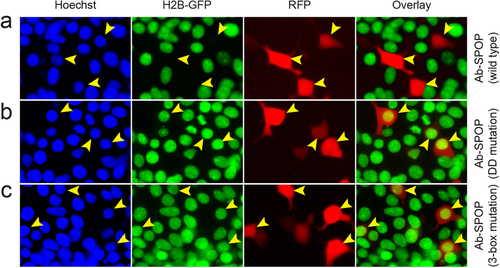
Ab-SPOPDDmutation and Ab-SPOP3-box mutation do not deplete nuclear H2B-GFP. 293TetOn cells expressing H2B-GFP were transfected with vector expressing Ab-SPOP, Ab-SPOPDDmutation, or Ab-SPOP3-box mutation from a bi-directional TRE promoter. (a) Ab-SPOP causes depletion of nuclear H2B-GFP in cells expressing TagRFP (yellow arrowheads). (b) Ab-SPOPDDmutation does not cause depletion of nuclear H2B-GFP in cells expressing TagRFP (yellow arrowheads). This suggests that the Ab-SPOP dimerization domain is necessary for the depletion of H2B-GFP. (c) Ab-SPOP3-box mutation does not cause depletion of nuclear H2B-GFP in cells expressing TagRFP (yellow arrowheads). This suggests that the Ab-SPOP 3-box domain is necessary for nuclear depletion of H2B-GFP. The 3-box domain is required for binding with Cul3 in the CRL3 E3 ligase complex14, indicating that Ab-SPOP interacts with Cul3 protein for its E3 ubiquitin ligase activity. |
|
Targeted depletion of Hmga2-Citrine in zebrafish embryos. (a) mRNA of wild type Ab-SPOP or mutant Ab(mut)-SPOP (deletion of CDR3 region of the vhhGFP4 nanobody), was injected into one-cell stage zebrafish embryos expressing Hmga2-Citrine protein (homozygous ct29aGT). TagRFP mRNA was co-administered as an injection control. Ab-SPOP expression abolishes Hmga2-Citrine, resulting in early developmental defects such as abnormal cell division during cleavage, delayed cell migration during epiboly and ultimately embryonic death. In contrast, embryos injected with Ab(mut)-SPOP mRNA did not affect Hmga2-Citrine level or disrupt embryonic development, suggesting that Ab-SPOP activity directly promotes degradation of Hmga2-Citrine protein resulting in developmental phenotypes. (b) The toxicity of Ab-SPOP was tested by injecting Ab-SPOP mRNA into wild type embryos (AB line). Embryonic development was normal. ‘n’ represents the total number of embryos from several injection experiments. |
|
Bi-directional doxycycline-inducible promoters allow simultaneous expression of synthetic E3 ligases and Myc10-TM (a) Each vector contains a bi-directional tetracycline response element (TRE) promoter, tetracycline repressor A3 (rtTA3), and TagRFP or Myc10-TM tracer (10 tandem repeats of Myc epitope-transmembrane domain). (b) Following doxycycline treatment, Myc10-TM was detected on the cell membrane by fluorescencent microscopy after immunostaining with anti-Myc primary antibody (9E10) and Alexa568-conjugated anti-mouse secondary antibody. |
|
Depletion of GFP in nucleus, but not in cytoplasm, by Ab-SPOP. 293TetOn cells expressing GFP were transfected with vectors expressing Ab-SPOPΔNLS or Abmutation-SPOPΔNLS a bi- directional TRE promoter. ′-′ indicates untransfected cells . Expression of TagRFP and depletion of GFP were measured after adding doxycycline (1 µg/ml) to media. TagRFP was first detected about 8 hours after induction of TRE promoters by doxycycline. Yellow arrowhead indicates cells in which GFP is depleted in the nucleus, but not in cytoplasm. Nuclear depletion of GFP started 3 hours after activation of TRE promoters and was only observed in cells transfected with Ab- SPOP. |
|
Selective depletion of nuclear GFP by Ab-SPOP E3 ligases engineered with other nanobodies against GFP. 293TetOn cells expressing GFP were transfected with vectors expressing four different Ab-SPOPΔNLS ligases (GBP1-SPOP, GBP2-SPOP, GBP6-SPOP, and GBP7- SPOP) from a bi-directional TRE promoter. Expression of TagRFP and depletion of GFP were measured 8 hours after adding doxycycline (1 µg/ml) to media. ′-′ indicates untransfected cells. Yellow arrowhead show cells where nuclear, not cytoplasmic GFP is depleted. |
|
Depletion of H2B-GFP by Ab-SPOP E3 ligases engineered with alternative GFP nanobodies. 293TetOn cells expressing H2B-GFP were transfected with vectors expressing four different Ab- SPOP ligases (GBP1-SPOP, GBP2-SPOP, GBP6-SPOP, and GBP7-SPOP) from the bi-directional TRE promoter. Expression of TagRFP and depletion of H2B-GFP were measured 8 hours after adding doxycycline (1 µg/ml) to media. ‘–‘ indicates untransfected cells. Blue signal in the merged Hoechst/H2B-GFP panel indicates cells with depleted nuclear H2B-GFP. All cells expressing TagRFP had depleted H2B-GFP, however some cells with depleted H2B-GFP did not express TagRFP, consistent with the observation that nuclear depletion begins 3 hours after activation of the TRE promoter (Supplementary Figs. 3 and 6). |
|
Time-course of H2B-GFP depletion by Ab-SPOP. 293TetOn cells expressing H2B-GFP were transfected with vectors expressing Ab-SPOP ligase (vhhGFP4-SPOP) or Abmutation-SPOP from bi- directional TRE promoters. Expression of TagRFP and depletion of nuclear H2B-GFP were measured after adding doxycycline (1 µg/ml) to media. Yellow arrowheads show nuclei with depletion of H2B-GFP. TagRFP was first detected about 8 hours after doxycycline-mediated TRE promoter activation. Depletion of nuclear H2B-GFP started 3 hours after TRE promoter activation and was observed in cells transfected with Ab-SPOP but not with AbmutationSPOP. |
|
Reversibility of Ab-SPOP. 293TetOn cells expressing H2B-GFP from a CMV promoter and Ab- SPOP/TagRFP from a bi-directional TRE promoter recover H2B-GFP signal after doxycycline withdrawal. (a) Cells grown for 60 hours in medium without doxycycline. (b) Cells grown for 60 hours in medium containing doxycycline (1 µg/ml). Medium was changed every 24 hours. (c) Cells treated with doxycycline (1 µg/ml) for 12 hours, and then changed to fresh medium containing no doxycycline. Medium was changed every 24 hours. After 48 hours in medium without doxycycline, the H2B-GFP signal was partially recovered. (d) Protein extracts from the cells treated with doxycycline (–: w/o doxycycline for 60 hours, +: w/ doxycycline for 60 hours, +: w/ doxycycline for 12 hours and then with fresh medium for 48 hours) were analyzed by immunoblotting with anti-GFP, anti-FLAG, and anti-Actin antibodies. |