- Title
-
Progenitor potential of nkx6.1-expressing cells throughout zebrafish life and during beta cell regeneration
- Authors
- Ghaye, A.P., Bergemann, D., Tarifeño-Saldivia, E., Flasse, L.C., Von Berg, V., Peers, B., Voz, M.L., Manfroid, I.
- Source
- Full text @ BMC Biol.
|
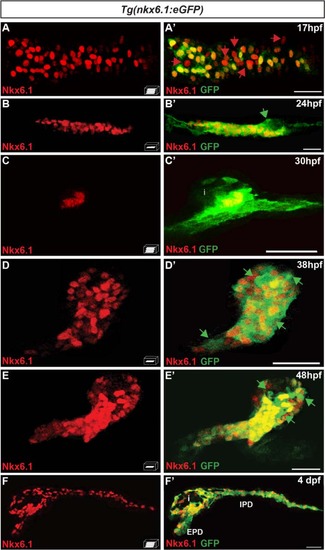
The bacterial artificial chromosome reporter line Tg(nkx6.1:eGFP) mirrors the expression of the endogenous nkx6.1 gene. Immunodetection of endogenous Nkx6.1 (red) and GFP (green) in Tg(nkx6.1:eGFP) embryos of the indicated stages. Green arrows point to Nkx6.1–/GFP+ cells and red arrows to Nkx6.1+/GFP- cells. All views are either lateral (b, b′, c, and c′) or ventral (a, a′, d, d′, f, and f′) with the anterior part to the left. They represent either z-plane confocal images (b, d, e) or confocal projection images (a, c, f). Scale bars = 30 µm. EPD extra-pancreatic duct, IPD intra-pancreatic duct, i islet EXPRESSION / LABELING:
|
|
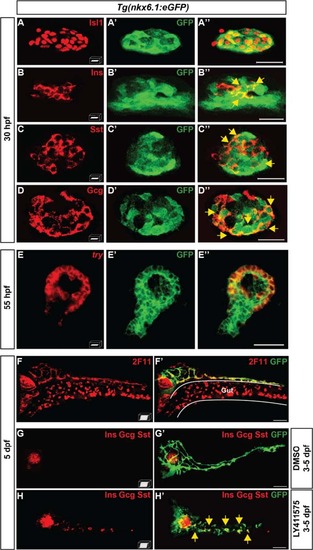
nkx6.1-expressing cells give rise to all pancreatic cell types. a–d" Immunodetection in 30-hpf Tg(nkx6.1:eGFP) embryos of GFP with Isl1 (a–a"), Ins (b–b"), Sst (c–c"), or Gcg (d–d"). Yellow arrows point to cells co-expressing GFP and the respective hormones. e–e" Fluorescent whole-mount in situ hybridization (WISH) of 55-hpf Tg(nkx6.1:eGFP) embryos using a try probe followed by GFP immunodetection. f, f′ Immunodetection in 5-dpf Tg(nkx6.1:eGFP) embryos of GFP and of the hepato-pancreatic duct marker 2F11. g–h′ Immunodetection of GFP and of Ins, Gcg, and Sst hormones in 5-dpf Tg(nkx6.1:eGFP) embryos treated from 3 to 5 dpf with dimethyl sulfoxide (DMSO) (g) or with the Notch signaling inhibitor, LY411575 (h). Yellow arrows point to secondary endocrine GFP+/hormones+ cells found in IPDs. All views are ventral with the anterior part to the left and represent either z-plane confocal images (a-e) or confocal projection images (f-h). Scale bars = 20 µm (a-e) or 40 µm (f-h) |
|
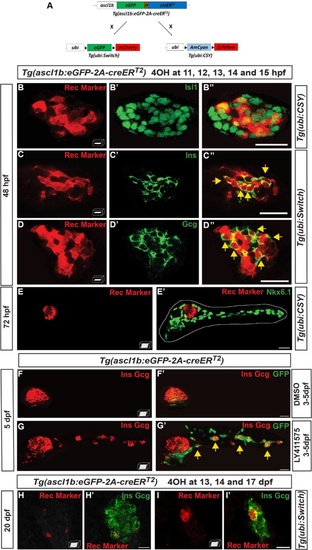
ascl1b-expressing cells give rise exclusively to endocrine cells of both dorsal and ventral bud. a–e′, h–i′ Genetic lineage tracing using the Cre-loxP system.a Schematic representation of the genetic lineage tracing experiments. The transgenic line Tg(ascl1b:eGFP-creER T2 ) was crossed with the Tg(ubi:loxP-eGFP-loxP-mCherry) line, abbreviated Tg(ubi:Switch), or with the Tg(ubi:loxP-AmCyan-loxP-ZsYellow) line, abbreviated Tg(ubi:CSY), treated with 4-hydroxytamoxifen (4OHT) at 11, 12, 13, 14, and 15 hpf (b–e′) or at 13, 14, and 17 dpf (h–i′) and fixed for analysis at the indicated times. Black triangles in a represent loxP sites. b–e′ Immunodetection of CRE-mediated rec markers (ZsYellow or mCherry, red) and Isl1 (b–b"), Ins (c–c"), Gcg (d–d"), or Nkx6.1 (e, e′) in 4OHT-treated embryos (green). The dotted white line delimits the pancreas (e′). Yellow arrows point to cells co-expressing rec marker (ZsYellow or mCherry) and the respective hormones (Ins or Gcg). f–g′ Short-term lineage tracing: immunodetection of GFP and the Ins and Gcg hormones in 5-dpf Tg(ascl1b:eGFP-creER T2 ) embryos treated from 3 to 5 dpf with DMSO (f, f′) or with the Notch signaling inhibitor, LY411575 (g, g′). Yellow arrows in g′ point to GFP+/Ins+/Gcg+ secondary endocrine cells found in the IPDs. h–i′ Immunodetection at 20 dpf of the CRE-mediated rec marker mCherry together with Ins and Gcg in 4OHT-treated larvae. All views are ventral with the anterior part to the left and represent z-plane confocal images (b–d") or confocal projection images (e–i′). Scale bars = 20 µm EXPRESSION / LABELING:
PHENOTYPE:
|
|
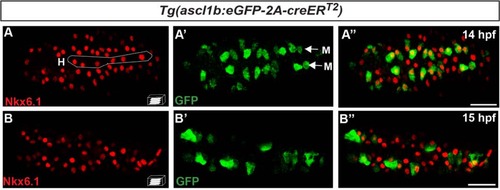
nkx6.1 and ascl1b are first co-expressed in the endocrine precursors of the dorsal bud but rapidly their expression domain moves apart. Immunodetection of endogenous Nkx6.1 and GFP in Tg(ascl1b:eGFP-creER T2 ) embryos at 14 hpf (a–a") and 15 hpf (b–b"). All views are ventral with the anterior part to the left and represent confocal projection images. Scale bars = 40 µm. H hypochord, M medial cells EXPRESSION / LABELING:
|
|
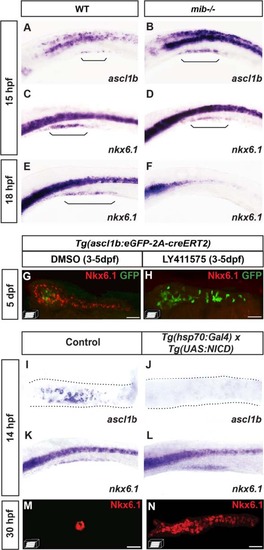
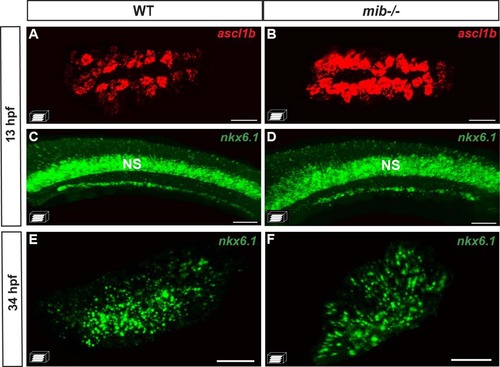
ascl1b and nkx6.1 are regulated in an opposite way by the Notch signaling pathway. a–f WISH on wild-type (WT) embryos (a, c, e) or mind bomb (mib-/-) mutants (b, d, f) with ascl1b (a, b) or nkx6.1 (c–f) probes. Brackets delimit the pancreatic domain. g, h Immunodetection of GFP and endogenous Nkx6.1 in 5-dpf Tg(ascl1b:eGFP-creER T2 ) embryos treated from 3 to 5 dpf with DMSO (g) or with the Notch signaling inhibitor, LY411575 (h). i–n WISH with ascl1b (i, j) or nkx6.1 (k, l) probes or immunodetection of endogenous Nkx6.1 (m, n) of double-transgenic Tg(hsp70:Gal4) x Tg(UAS:NICD) embryos (j, l, n) or of control simple-transgenic embryos (i, k, m), both heat-shocked at 11 hpf for 20 min. Lateral views (a–f, k, l) or ventral views (g–j, m, n) of embryos of the indicated stages with the anterior to the left. Scale bars = 40 µm EXPRESSION / LABELING:
PHENOTYPE:
|
|
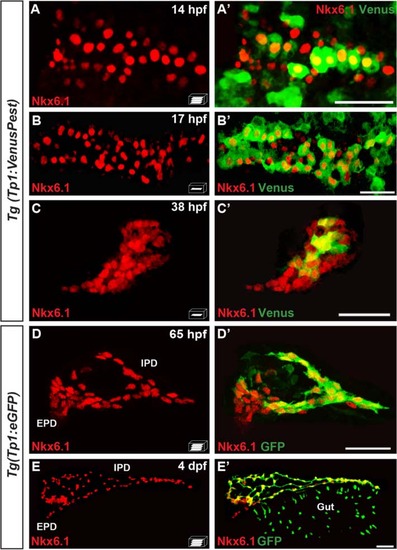
The pancreatic expression domain of Nkx6.1 includes the Notch-responsive cells. Immunodetection of endogenous Nkx6.1 (red) and Venus (revealed with anti-GFP, green) in Tg(TP1:VenusPest) (a–c′) or GFP in Tg(Tp1:eGFP) embryos (d–e′) at the indicated stages. All views are ventral with the anterior part to the left and represent either z-plane confocal images (b–c′) or confocal projection images (a, a′, d, d′, e, e′). Scale bars = 40 µm. EPD extra-pancreatic duct, IPD intra-pancreatic duct EXPRESSION / LABELING:
|
|
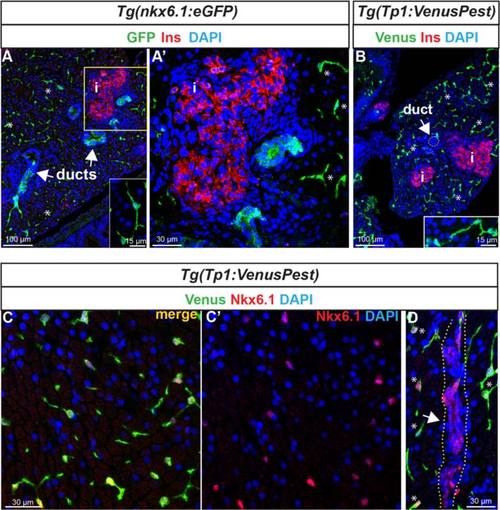
Expression of nkx6.1 persists in duct cells in the adult pancreas. a GFP and insulin (Ins, red) immunodetection on section through the pancreas of adult Tg(nkx6.1:eGFP) zebrafish. White arrows point to pancreatic ducts and asterisks show cells (presumably centroacinar/ terminal end duct cells (CACs) dispersed throughout the exocrine tissue). a′ Close-up of the islet highlighted with Ins. b Venus (green) and Ins (red) immunodetection in Tg(Tp1:VenusPest) showing the presence of Venus in CACs (asterisks) as previously reported 12], but not in duct cells within ductular structures (white arrow). Insets in (a) and (b) show isolated CACs. c–d Immunodetection of Venus (green) and of endogenous Nkx6.1 (red) in Tg(Tp1:VenusPest) revealing co-labeling of both markers in CACs (c, c′) while Nkx6.1 alone, but not Venus, labels the ducts. The white arrow in (d) points to a duct and asterisks indicate CACs. Dotted yellow lines delimit the duct. c′ Same as (c) showing Nkx6.1 (red) and 4′,6-diamidino-2-phenylindole (DAPI) only. i, islet EXPRESSION / LABELING:
|
|
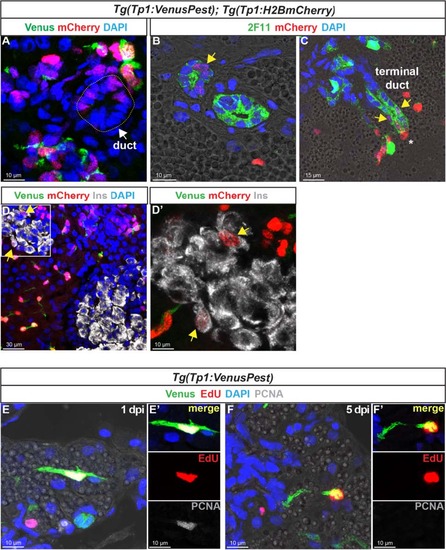
In the adult pancreas, Notch-responsive CACs give rise to other ductal and endocrine cells and have the capacity to replicate. Immunodetection on sections through the pancreas of adult Tg(Tp1:VenusPest); Tg(Tp1:H2BmCherry).a Comparison of Venus (green) and H2BmCherry (red) labeling showing a small duct containing H2BmCherry+ cells that have lost Venus. b Comparison of H2BmCherry (red) with the ductal marker 2F11 (green) showing some 2F11+ cells within a small duct co-expressing the stable H2BmCherry marker (yellow arrow). c Weak H2BmCherry labeling near the extremity of a ductular structure (terminal or intercalated duct) (yellow arrows); a CAC (intense H2BmCherry) at the tip of the terminal duct is indicated by an asterisk. d and d′ (close-up) Some H2BmCherry+ cells, devoid of Venus (Notch off) co-express the beta cell marker Ins (white) (yellow arrows). Sections acquired in the head of the pancreas, at the level of the main endocrine islet. e–f′ Detection of 5-ethynyl-22-deoxyuridine (EdU) (red), Venus (green), and proliferating cell nuclear antigen (PCNA) (white) in Tg(Tp1:VenusPest) adult fish injected with EdU. The pancreas was analyzed 20 hours (1 day post injection) (e, e′) and 5 days (f, f′) after EdU injection EXPRESSION / LABELING:
|
|
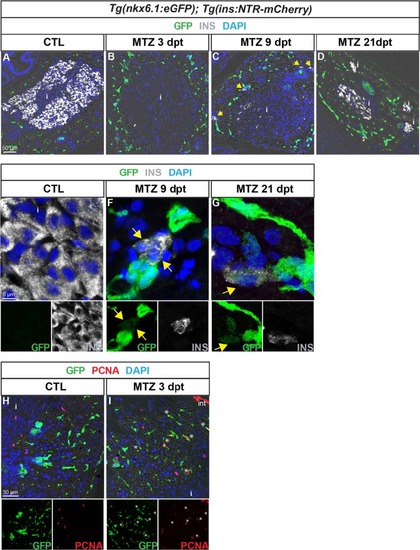
nkx6.1:eGFP+ cells proliferate and differentiate into new insulin-expressing cells after beta cell specific ablation. GFP (green) and Ins (white) labeling in the pancreas of Tg(nkx6.1:eGFP); Tg(ins:NTR-mCherry) adult fish. a, b Non-treated fish (CTL, a) show intense Ins staining in beta cells, while Ins+ cells are not detected 3 dpt with MTZ, indicating efficient ablation (b). Note that debris of one beta cell (Ins+) is observed in the islet. c At 9 dpt, Ins-expressing cells start to be detected in the principal islet and in extra-insular locations close to ductal GFP+ cells (yellow arrows). d At 21 dpt, islets show intense Ins staining consistent with beta cell recovery. e–g (and separate channels) While GFP is never detected in beta cells of control fish (e), some regenerating Ins+ cells display weak GFP labeling at both stages of regeneration analyzed, i.e. at 9 dpt (f) and 21 dpt (g). h, i (and separate channels) Beta cell ablation triggers proliferation of CACs as shown at 3 dpt (h) compared to CTL (i) (asterisks). i islet |
|
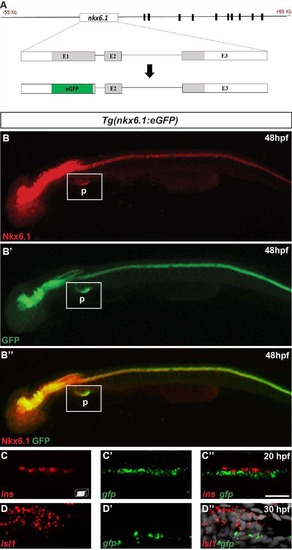
The bacterial artificial chromosome (BAC) reporter line Tg(nkx6.1:eGFP) mirrors the expression of the endogenous nkx6.1 gene. A Schematic representation of the 55 to +95 kb nkx6.1:eGFP BAC transgene. This BAC includes sequence blocks that are highly conserved amongst vertebrates (black boxes) located from 11 to 77 kb downstream of the nkx6.1 gene. By BAC recombineering using galK selection, the eGFP cassette (green box) was introduced into exon 1, replacing the beginning of the nkx6.1 open reading frame (aa 1 to 149). B Epifluorescence microscopy images of the immunodetection of endogenous Nkx6.1 (red) and GFP (green) in Tg(nkx6.1:eGFP) embryos. Lateral views of 48 hpf embryos with the anterior part to the left. C Confocal projection images of whole mount fluorescent in situ hybridization on a Tg(nkx6.1:eGFP) embryo showing that the gfp transcripts are not expressed in the insulin+ cells at 20 hpf. D Confocal projection images of whole mount fluorescent in situ hybridization on a Tg(nkx6.1:eGFP) embryo showing that the gfp transcripts are not expressed in the isl1+ cells at 30 hpf. Scale bar = 15 µm. P pancreas. |
|
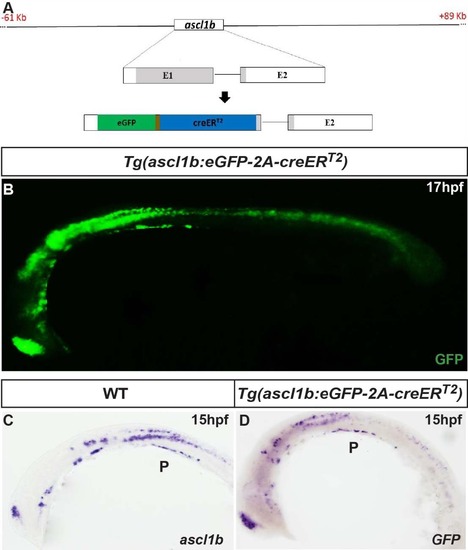
The bacterial artificial chromosome (BAC) reporter line Tg (ascl1b:eGFP-creER T2 ) mirrors the expression of the endogenous ascl1b gene. A Schematic representation of the 61 to +89 kb ascl1b:eGFP- 2A-creER T2 BAC transgene. By BAC recombineering using galK selection, the eGFP-2A-creER T2 cassette is introduced into exon 1, replacing the beginning of the ascl1b open reading frame (aa 1 to 163). B Epifluorescence microscopy images of the immunodetection of GFP in 17-hpf Tg(ascl1b:eGFP-creER T2 ) embryos. C, D Visible WISH showing expression of endogenous ascl1b in a wild-type (WT) embryo (C) and of GFP in Tg(ascl1b:eGFP-creER T2 ) embryos (D) at 15 hpf. Lateral views with the anterior part to the left. P pancreas. |
|
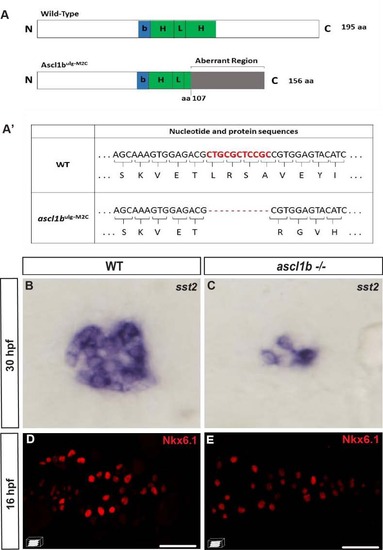
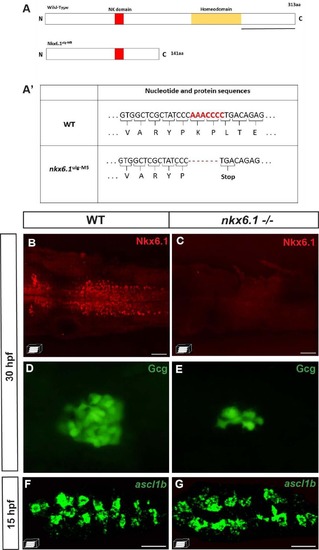
nkx6.1 expression is not repressed by Ascl1b. A Schematic representation of wild-type (WT) and mutant Ascl1b ulg-M2C proteins. The basic domain (b) (+70/+78) is represented by a blue box and the helix loop helix (HLH) domain (+79/+123) by a green box. The coding region of the mutant Ascl1b ulg-M2C protein contains an 11-bp deletion after the aa 107 codon, leading to a frameshift and the production of an aberrant region of 48 aa instead of the second helix, known to be essential for the function of the bHLH proteins. A′ Table showing part of the nucleotide and protein sequence of the ascl1b gene and of the mutated form in the Ascl1b ulg-M2C mutant. B, C WISH showing the drastic reduction of somatostatin expression in the ascl1b -/- mutant compared to the WT embryo at 30 hpf. This phenotype is identical to the one of embryos injected with a translation-blocking morpholino, targeting the translation start site of ascl1b mRNA [15], [77], [78], suggesting that the mutant Ascl1b ulg-M2C is effectively a null mutant. D, E Confocal projection images of Nkx6.1 immunodetection showing equivalent number of nkx6.1+ cells in WT and ascl1b-/- mutants at 16 hpf. All views are ventral with the anterior part to the left. Scale bars = 40 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
ascl1b expression is not repressed by Nkx6.1. A Schematic representation of wild-type (WT) and mutant Nkx6.1 ulg-M5 proteins with the yellow box representing the homeodomain and the red box representing the NK domain. The coding region of the mutant Nkx6.1 ulg-M5 protein contains a 7-bp deletion after the aa 141 codon, leading to the apparition of a STOP codon just after the deletion. The black line represents the region of the protein recognized by the Nkx6.1 antibody. A′ Table showing part of the nucleotide and protein sequence of the nkx6.1 gene and of the mutated form in the Nkx6.1 ulg-M5 mutant. B, C Nkx6.1 immunodetection showing the loss of the full-length Nkx6.1 protein in the nkx6.1 -/- mutant. D, E Glucagon immunodetection showing a drastic reduction of the number of glucagon-expressing cells in the nkx6.1-/- mutant compared to the WT embryo at 30 hpf. This phenotype is the same as the one of embryos injected with the MO1 translation-blocking morpholino, targeting the translation start site of nkx6.1 mRNA [18], shown to prevent nkx6.1 expression efficiently in the neural tube [72]. This strongly suggests that the mutant Nkx6.1 ulg-M5 is a null mutant. F, G Confocal projection images of fluorescent WISH showing equivalent number of ascl1b+ cells in WT and nkx6.1-/- mutants at 15 hpf. All views are ventral with the anterior part to the left. Scale bars = 40 µM. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The initiation of nkx6.1 expression is independent of Notch signaling. A–D Fluorescent WISH showing a drastic increase in the pancreatic dorsal bud of ascl1b expression in the mind bomb (mib -/- ) mutants while nkx6.1 expression is not affected at 13 hpf. E, F Fluorescent WISH showing that the pancreatic expression of nkx6.1 in the ventral bud is not perturbed at 34 hpf in mib -/- mutants. The ventral (A, B, E, F) or lateral (C, D) views represent confocal projection images with the anterior to the left. Scale bars =40 µM. NS nervous system. |
|
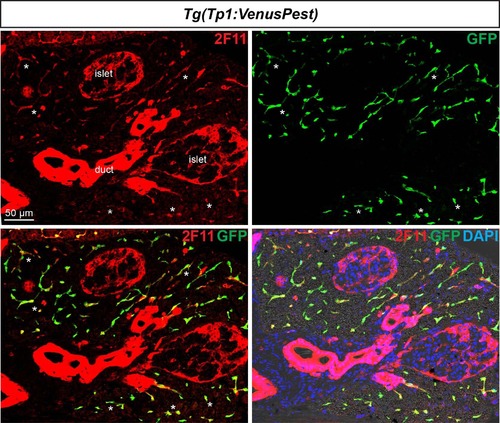
All Notch-responsive cells in the adult pancreas are CACs. Notch active cells in Tg(Tp1:VenusPest) adult fish were labeled with anti-GFP to visualize VenusPest (green) and the ductal marker 2F11 (red). VenusPest labeling is detected throughout the exocrine tissue (see asterisks highlighting examples) and where it highlights the CACs together with 2F11. In contrast, ductal structures with intense 2F11 staining do not exhibit any Venus+ (Notch on) cells. Note also the presence of 2F11 in endocrine islet cells as previously reported [79]. |
|
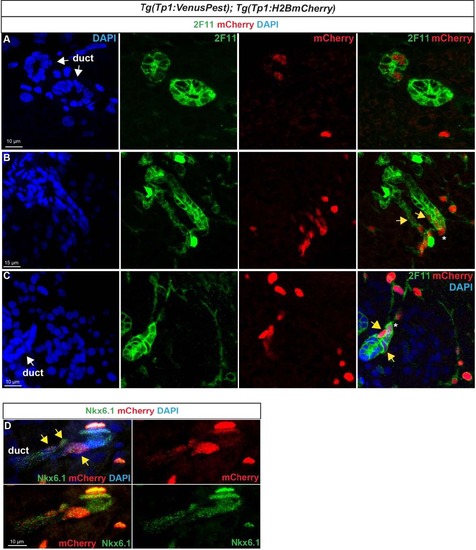
Notch-responsive terminal end duct cells/CACs give rise to other ductal cells. Immunodetection of H2BmCherry and ductal markers in the pancreas of adult Tg(Tp1:VenusPest); Tg(Tp1:H2BmCherry) zebrafish.A–C H2BmCherry+ cells (red) in ductular structures labeled with the ductal (ducts and CACs) marker 2F11 (green). A, B separate channels of Fig. 8b–c. B, C Weak H2BmCherry labeling is present near the extremity of a terminal (or intercalated) duct (yellow arrows). The asterisks identify a CAC at the tip of the duct (strong H2BmCherry labeling). D Comparison of H2BmCherry (red) with endogenous Nkx6.1 (green) showing ductal Nkx6.1+ cells co-expressing H2BmCherry (yellow arrows). |
|
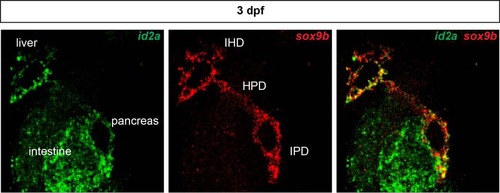
Ductal expression of id2a during zebrafish development. Whole mount fluorescent in situ hybridization showing id2a (green) and the ductal marker sox9b (red) in a 3-dpf larva. The expression of both genes overlaps as highlighted by yellow labeling. In addition to expression in the intrapancreatic ducts (IPD), id2a is also expressed in the intrahepatic (IHD). HPD hepatopancreatic ducts. |