- Title
-
Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish
- Authors
- Evason, K.J., Francisco, M.T., Juric, V., Balakrishnan, S., Lopez Pazmino, M.D., Gordan, J.D., Kakar, S., Spitsbergen, J., Goga, A., Stainier, D.Y.
- Source
- Full text @ PLoS Genet.
|
Hepatocyte-specific expression of activated β-catenin results in liver enlargement, hepatocellular carcinoma (HCC), and decreased survival in adult zebrafish. (A-C) Control 4-month-old zebrafish showing the liver (L, outlined) positioned in the body cavity near the intestine (i) and swim bladder (sb)(A). Sections show an orderly arrangement of hepatocytes (B-C). (D-F) Transgenic 4-month-old zebrafish showing an enlarged liver (D) with disorganized architecture (E) and atypical cells (arrows, F). (G-H) Transgenic 6-month-old (G) or 4-month-old (H) zebrafish and human HCC showing architectural disruption with scattered pseudoglands (arrows, G) and evidence of intracellular lipid accumulation (arrows, H). Hematoxylin and eosin stains; scale bars, 1 mm (A, D), 100 µm (B, E), 25 µm (C, F), and 20 µm (G, H). (I) Graph showing average liver size normalized to total body mass, ± standard error of the mean (SEM). Asterisks indicate p-values for ANOVA comparing transgenic zebrafish (N = 56) to control siblings (N = 51) at the same time point: *, p<0.05; ***, p<0.001. (J) Livers of transgenic zebrafish (Tg, N = 49) and control siblings (C, N = 37) were examined microscopically, and architectural and cytological changes were scored. HCC was significantly more common in transgenic zebrafish than in controls (p<0.001, Fisher’s exact test). (K) Kaplan-Meier survival curves comparing adult survival of transgenic zebrafish (N = 51) and control siblings (N = 85); p<0.001, logrank test. PHENOTYPE:
|
|
Activated β-catenin causes larval liver enlargement and increased hepatocyte proliferation. (A) Brightfield images of control sibling and transgenic 6-day-old fixed larvae. Livers are outlined. Scale bars, 100 µm. (B) Graph showing average liver size ± SEM of 6-day-old larvae from three different transgenic lines (s985, s986, s987) compared to control siblings (C). N values are shown above the x-axis. Asterisks indicate p-values for 2-way ANOVA comparing transgenic zebrafish to control siblings in the same experiment: **, p<0.01; ***, p<0.001. (C) Immunofluorescence images of 6-day-old control and transgenic larvae, highlighting hepatocyte cell membranes (Tg(fabp10a:rasGFP)) and proliferating cells (EdU). Scale bars, 20 µm. Inset photos are 4X magnifications. (D-E) Graphs showing average cell size ± SEM (D) and percent of hepatocytes that were EdU positive (E). N values, which are the same for both graphs, appear above the x-axis in D; samples were compared using the Student’s t-test. *, p<0.05. |
|
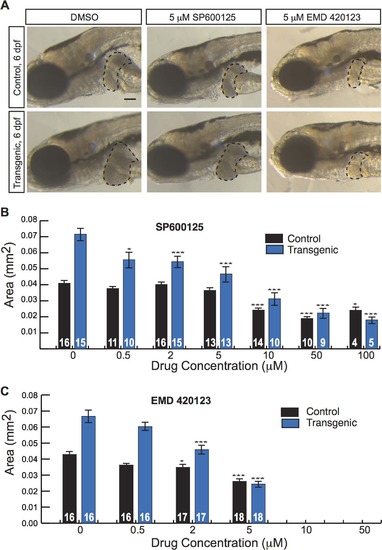
JNK inhibitors suppress larval liver enlargement caused by activated β-catenin. (A) Brightfield images of control sibling and transgenic 6-day-old fixed larvae, treated with DMSO or JNK inhibitors. Livers are outlined. Scale bar, 100 µm. (B-C) Graphs showing average liver size ± SEM of 6-day-old control sibling and transgenic larvae treated for 3 days with SP600125 (B) or EMD 420123 (C) at the indicated dosages. N values are shown above the x-axis. Asterisks indicate p-values for 2-way ANOVA comparing drug-treated zebrafish to DMSO-treated siblings with the same genotype: *, p<0.05; ***, p<0.001. PHENOTYPE:
|
|
Activated β-catenin is associated with JNK pathway activation. (A) Representative whole-mount immunofluorescence images and (B) quantification of 5-day-old control sibling and transgenic larvae treated for 2 days with 0.5% DMSO alone, 5 µM SP600125, or 5 µM EMD 420123 and stained with antibodies against phospho-c-Jun (left panels) and TO-PRO nuclear stain (right panels). Livers are outlined in white. Scale bar, 20 µm. N values are shown above the x-axis. Drug-treated zebrafish were compared to DMSO-treated siblings with the same genotype using 2-way ANOVA. ***, p<0.001. (C) Representative photographs of zebrafish control and Tg(fabp10a:pt-β-cat) livers (left) and human HCC without (middle) and with (right) activated β-catenin, stained with anti-phospho-c-Jun antibodies. For zebrafish, 6 out of 8 (75%) Tg(fabp10a:pt-β-cat) livers with HCC contained one or more foci with moderate or high nuclear phospho-c-Jun staining (phospho-c-Jun positive), while all non-transgenic control livers (N = 7) without activated β-catenin showed absent or low staining (phospho-c-Jun negative). For human HCC, the number of cases exhibiting each staining pattern and the total number of cases with a given β-catenin activation status are shown at the bottom right of each picture. Images counterstained with hematoxylin. Scale bars, 20 µm. (D) Normalized jun mRNA expression in control sibling and transgenic adult zebrafish livers. Three technical replicates were performed for each sample. *, p<0.05, Mann-Whitney test. N values are shown above the x-axis. (E) Normalized JUN mRNA expression for human HCC with low GLUL and high GLUL expression. ***, p<0.001, Mann-Whitney test. (F) Graph showing dose of JNK inhibitor CC401 at which cell viability of human cancer cell lines was decreased by 50% (GI50), ± SEM. Number of replicates for each cell line is shown above the x-axis. *, p<0.05, unpaired t-test. |
|
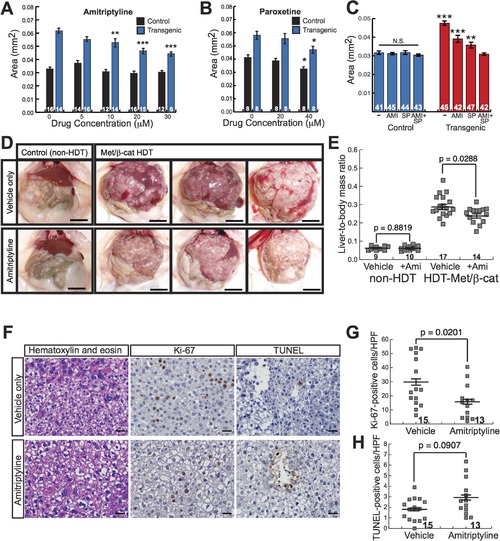
Antidepressants decrease β-catenin-induced liver enlargement and tumorigenesis. (A-B) Graphs showing mean liver size ± SEM of 6-day-old control sibling and transgenic zebrafish larvae treated for 3 days with amitriptyline (A) or paroxetine (B) at the indicated dosages. N values are shown above the x-axis. Asterisks indicate p-values for 2-way ANOVA comparing drug-treated zebrafish to DMSO-treated siblings with the same genotype: *, p<0.05; **, p<0.01; ***, p<0.001. (C) Graph showing mean liver size ± SEM of 6-day-old control sibling and transgenic zebrafish larvae treated for 3 days with 0.5% DMSO (-), 20 µM amitriptyline (AMI), 2 µM SP600125 (SP), or both drugs combined (AMI+SP). N values are shown above the x-axis. Asterisks indicate p-values for 2-way ANOVA comparing each group of transgenic zebrafish to AMI+SP group: **, p<0.01; ***, p<0.001. N.S., no significant difference between groups of non-transgenic zebrafish (2-way ANOVA). (D) Representative images of control, non-hydrodynamically transfected (non-HDT) mouse livers (left) and mouse liver tumors induced by hydrodynamic transfection of activated β-catenin and Met (Met/β-cat HDT). Mice were treated with saccharine alone (vehicle only, top row) or amitriptyline plus saccharine (bottom row). Scale bars, 1 cm. (E) Graph showing mean liver-to-body mass ratios ± SEM for non-HDT and HDT-Met/β-cat mice treated with saccharine alone (vehicle) or amitriptyline plus saccharine (+Ami). P values calculated with Mann-Whitney test. N values are shown above the x-axis. (F) Representative hematoxylin-and-eosin-stained, Ki-67-labeled, and TUNEL-labeled images from vehicle- and amitriptyline-treated mice. Ki-67 and TUNEL stainings were performed using 3, 3′-diaminobenzidine (DAB) substrate, so positive-staining cells are brown, and hematoxylin counterstain to highlight nuclei and other basophilic structures in blue. (G-H) Graphs showing mean ± SEM of Ki-67-positive (G) or TUNEL-positive (H) cells per high-power field. P values calculated with Mann-Whitney test. N values are shown above the x-axis. |
|
Tg(fabp10a:pt-β-cat) zebrafish express activated β-catenin. (A) Plasmid encoding hepatocyte-specific pt-β-catenin and a fluorescent eye marker was injected into embryos to generate Tg(fabp10a:pt-β-cat, cryaa:Venus) zebrafish. (B-C) Normalized transgenic Xenopus (B) and endogenous zebrafish (C) ctnnb1 mRNA expression in control and transgenic adult zebrafish livers. Three technical replicates were performed for each sample. Groups were compared using Mann-Whitney test. N values are shown above the x-axis. (D) Immunofluorescence of adult control sibling zebrafish liver cryosections (top panels) show membrane localization of β-catenin, whereas transgenic zebrafish livers show patchy cytoplasmic β-catenin staining and scattered β-catenin-positive nuclei (arrows). (E) Similarly, whole-mounted 5-day-old control sibling larvae (top row) show membrane localization of β-catenin while transgenic zebrafish (bottom row) show patchy strong cytoplasmic and nuclear β-catenin staining. Immunofluorescent staining for β-catenin was performed in Tg(fabp10a:EGFP) larvae, which have green hepatocytes; merged images show β-catenin is expressed in hepatocytes. (F) Six-day-old control sibling larvae (left) show essentially no 7xTCF-Xla.Siam:GFP (Wnt reporter) expression in hepatocytes, while transgenic larvae (right) show scattered hepatocytes with moderate to strong GFP positivity. (G) Fluorescence intensity ± standard error of the mean (SEM) was quantified using ImageJ, and samples were compared using the Student’s t-test (***, p<0.001). Scale bars, 20 µm. N values are shown above the x-axis. |
|
Zebrafish livers with activated β-catenin share histologic features with human hepatocellular carcinoma (HCC). (A) Adult zebrafish livers with activated β-catenin (left) and human HCC (right) show architectural disruption including enlarged interhepatic spaces resembling spongiosis hepatis (zebrafish) or peliosis hepatis (human) (asterisks). (B-D) Similarly, zebrafish (left) and human (right) samples show cytological abnormalities including nuclear enlargement and nuclear contour irregularities (arrows, B), prominent nucleoli (arrows, C), and mitotic figures (arrows, D). Hematoxylin and eosin (H&E) stained sections; scale bars, 20 µm. |
|
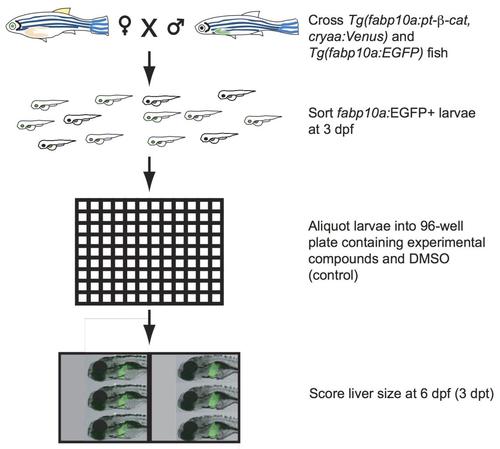
Small molecule screen for compounds that suppress larval liver enlargement caused by activated β-catenin. Transgenic zebrafish expressing activated β-catenin (Tg(fabp10a:pt-β-cat, cryaa:Venus)) were crossed to zebrafish expressing liver-specific GFP (Tg(fabp10a:EGFP). At 3 days old, larvae with green livers (fabp10a:EGFP+) were selected for drug treatment. Zebrafish with activated β-catenin, identified by their fluorescent eyes, were treated in parallel to control zebrafish without activated β-catenin. Three zebrafish were placed in each well, and 4 wells were tested for each experimental compound and vehicle (DMSO) control. Liver size was scored qualitatively 3 days post treatment (dpt). Drugs that decreased average liver size of Tg(fabp10a:pt-β-cat); Tg(fabp10a:EGFP) zebrafish in both wells compared to DMSO controls without causing toxicity/death in any wells were considered potential hit compounds. |
|
SP600125 and amitriptyline do not significantly affect fabp10a promoter activity or Wnt reporter activity. (A) Representative photographs of fabp10a:EGFP expression (the same fabp10a promoter element used to drive pt-β-cat expression) in control (top row) and Tg(fabp10a:pt-β-cat) (bottom row) zebrafish livers at 5 dpf. (B) Fluorescence intensity ± standard error of the mean (SEM) was quantified using ImageJ, and samples were compared using 2-way ANOVA; p>0.05 for each group compared to every other group. N values are shown above the x-axis. (C) Representative photographs of 7xTCF-Xla.Siam:GFP (Wnt reporter) expression in control (top row) and Tg(fabp10a:pt-β-cat) (bottom row) zebrafish livers at 6 dpf. (D) Fluorescence intensity ± standard error of the mean (SEM) was quantified using ImageJ, and samples were compared using 2-way ANOVA (p>0.05 for all drug treatments compared to DMSO control of same genotype.) Scale bars, 20 µm. Six zebrafish were analyzed for each group. |