- Title
-
Human Kidney Disease-causing INF2 Mutations Perturb Rho/Dia Signaling in the Glomerulus
- Authors
- Sun, H., Al-Romaih, K.I., MacRae, C.A., Pollak, M.R.
- Source
- Full text @ EBioMedicine
|
INF2 fish expression in zebrafish as detected by in situ hybridization and immunohistochemistry. a. The transcription of zINF2 at different stages of development was illustrated by using in situ hybridization (sense probe serving as a control). b. Detailed localization of INF2 transcription in wt zebrafish. c. A transverse section at kidney plane shows INF2 mRNA transcription (sense probe as a control). Immunohistochemistry stain shows INF2 expression in zebrafish at sagittal, transverse sections (d) and at kidney plane (PBS instead of 1st antibody was used in control sections). NS: nervous system; L: liver; G: Glomerulus; T: tubules. EXPRESSION / LABELING:
|
|
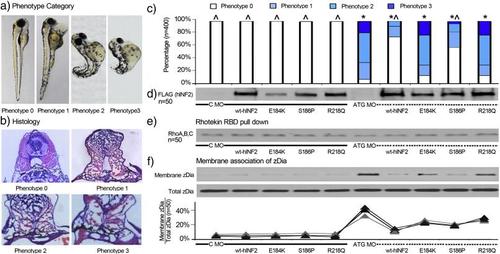
Zebrafish phenotype associated with INF2 expression and Dia activity. Control MO and INF2 ATG MO and in vitro transcribed hINF2 mRNAs were co-injected into zebrafish embryos at the single-cell stage. a, b, c. 400 zebrafish expressing different forms of INF2 (50 per group) were categorized and quantified based on different gross phenotype and histology at 96 h post fertilization (hpf). Phenotype 0: grossly and histologically identical with wt fish at 96 hpf; phenotype 1: significant pericardia edema, slightly disorganized sarcomeres at the tip of the tails, without significant tail curling or dorsalization. Histologically collapsing or underdeveloped glomerular tufts.; phenotype 2: Diffuse edema with prominent yolk, significant bending and dorsalization. Histologically, underdeveloped glomerulus without open capillary tuft.; phenotype 3: dorsalized embryo with shortened tail, diffuse edema, underdeveloped pronephros, and distended tubules. Statistical significance, χ2 test: *, p < 0.05 vs. C-MO only; ^, p < 0.05 vs. ATG-MO only. d. Expression of hINF2 protein (Flag-tagged) was confirmed by western blot in 50 zebrafish in each group. e. Rho activity in zebrafish with expression of different forms of INF2 was measured by Rhotekin RBD pulldown assay. f. Dia activity in zebrafish with different manipulations of INF2 was measured and quantified as the ratio of membranous associated Dia2 (Membrane/Total Dia2). PHENOTYPE:
|
|
INF2 knockout leads to altered podocyte structure and nephrin mistrafficking. a. Ultrastructure of glomerulus and podocyte in INF2 morphant zebrafish vs. control fish. b. Cultured podocytes were transfected with INF2-targeting siRNA or a control RNA duplex (control siRNA) for 48 h. Then the cells in both groups were transfected with a plasmid encoding human nephrin. 24 h later, nephrin expression was illustrated by immunofluorescence stain in cultured podocyte with INF2 knockdown (INF2 siRNA) vs. control cells (control siRNA). c. Glomerular section of INF2 morphants (INF2 MO) and control fish (control MO). The expression of nephrin is illustrated using immunofluorescence staining in the zebrafish glomerulus (INF2 morphants (INF2 MO) vs. control fish (control MO)). |
|
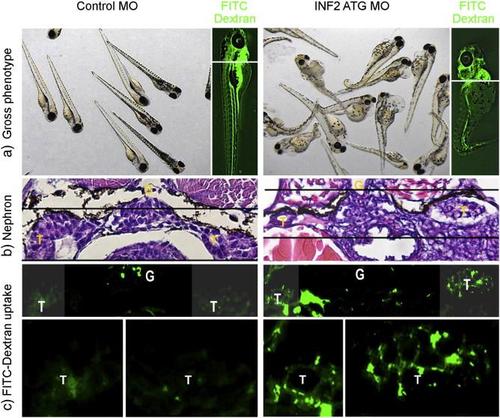
INF2 knockout in zebrafish results in slit diaphragm dysfunction. Control and INF2 morphants were examined for the effect of INF2 knockdown on the gross phenotype (a) and histology of glomerulus (G) (b). At 96 hpf, FITC-labeled dextran was injected into the cardinal vein of the fish. 24 h later, fish were sacrificed and sections at the kidney plane were examined under light microscopy (b) and fluorescence microscopy (c). Uptake of injected FITC-dextran in tubular cells indicates the increased leakage of molecules through the glomerular filtration barrier with INF knockdown (d. magnified tubular cells, INF2 MO vs. Control MO). G denotes glomerulus, T denotes tubule. PHENOTYPE:
|
|
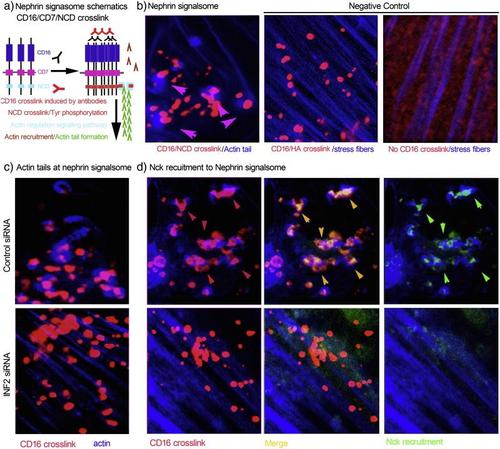
INF2 depletion in cultured podocytes destroys the integrity of slit diaphragm signaling. a. Schematic of CD16/Nephrin cytoplasmic domain (NCD) crosslink model. In cultured human podocytes expressing recombinant CD16/CD7/NCD, anti-CD16 antibody and Alexa Fluor594-conjugated secondary antibody were applied to induce CD16/NCD crosslink. The crosslink leads to phosphorylation of Tyr residuals in NCD, recruitment of Nck and actin remodeling. b. The successful crosslink exhibits blue actin tails attached to red nephrin signalsome particles. Podocytes expressing CD16/CD7/HA with CD16 crosslinked (no actin tails attaching to CD16/HA crosslink particles) and podocytes expressing CD16/CD7/NCD without CD16 crosslinked served as two negative controls. c. In podocytes expressing CD16/CD7/NCD, INF2 knockdown disrupts GFP-Nck recruitment (d) the actin tail formation (c and d) despite successful crosslinking, leading to loss of normal of slit diaphragm signaling. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) |
|
Co-injection of Rhoa or Dia2 morpholinos rescues the INF2 morphant phenotype. 100 surviving zebrafish embryos in each group (control MO, INF2 MO, Rhoa MO, INF2 + Rhoa MO, Dia MO, INF2 + Dia MO) were randomly selected. The survival rate (a) and the phenotype distribution (b; phenotype 0, 1, 2, 3 as defined above) were recorded at different stages of development (48 h, 72 h, 96 h, 5 d, and 7 d). The data was collected in 3 independent experiments. Distribution of the gross zebrafish phenotypes at 72 hpf, as defined in Fig. 3. PHENOTYPE:
|