- Title
-
Polo-like kinase 2 regulates angiogenic sprouting and blood vessel development
- Authors
- Yang, H., Fang, L., Zhan, R., Hegarty, J.M., Ren, J., Hsiai, T.K., Gleeson, J.G., Miller, Y.I., Trejo, J., Chi, N.C.
- Source
- Full text @ Dev. Biol.
|
PLK2 is expressed in endothelial cells.(A–B′′) Immunostaining of HUVECs reveals that PLK2 is expressed in ECs. (B–B′′) Enlarged image of the boxed area in A–A′′ shows that PLK2 can aggregate at the leading edge of extending ECs (arrowhead). (A and B)-merge; (A′ and B′)-anti-PLK2 immunostaining (green); (A′′ and B′′)-phalloidin actin staining (red). DAPI nuclear staining (blue). Scale bar, 40 µm. (C) Quantitative measurements of PLK2 localized at the leading edge and in the cytoplasm of HUVECs (Mean±s.e.m. *p=0.0056 by Student′s t-test). (D–F) in situ hybridization shows that plk2b is expressed in the zebrafish vasculature at 16 hpf (n=31/31), 24 hpf (n=46/47), and 48 hpf (n=28/30). (D′–F′) Enlarged image of the boxed area of D–F shows that plk2b is expressed in the cardinal vein, aorta, and intersomitic vessels of the zebrafish body and tail. EXPRESSION / LABELING:
|
|
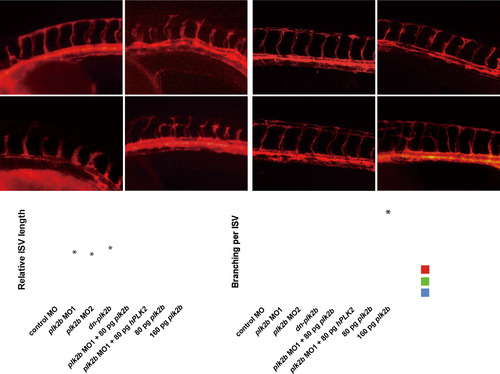
plk2b regulates endothelial cell sprouting. (A–D) Fluorescence micrographs show that loss of plk2b function in Tg(kdrl:mcherry-ras) zebrafish embryos results in underdeveloped intersomitic vessel sprouting (open arrowheads) at 36 hpf. (A) control MO (n=0/149); (B) plk2b ATG MO (MO1) (n=125/171); (C) plk2b splice MO (MO2) (n=89/137); (D) dn-plk2b RNA – dominant negative plk2b (n=49/85). Injecting (E) 80 pg of plk2b (n=31/103) or (F) 80 pg of hPLK2 (n=24/71) can rescue the plk2b MO1 vascular sprouting defect. (G) Injecting 80 pg of plk2b RNA into wild-type Tg(kdrl:mcherry-ras) embryos did not cause any significant vascular phenotypes (n=0/105). However, (H) injecting 160 pg of plk2b resulted in increased ISV spouting and branches (arrowheads) (n=91/145). Top, dorsal longitudinal anastomotic vessel (yellow asterisk); bottom, dorsal aorta/cardinal vein. Scale bar, 80 µm. Quantitative measurements of (I) intersomitic vessel length and (J) the number of ISV branches for each corresponding condition. Mean±s.e.m. *p<0.05 by ANOVA. |
|
PLK2 regulates endothelial cell lamellipodia and cell adhesion formation. (A–A′) 26 hpf plk2b MO1 injected Tg(fli1a:eGFP) fish (n=31/40) exhibit fewer lamellipodia, and extending intersomitic vessels compared to age-matched control MO injected Tg(fli1a:eGFP) fish (n=0/48). Scale bar, 40 µm. Top, dorsal longitudinal anastomotic vessel; bottom, dorsal aorta/cardinal vein. (B–B′) Phalloidin staining reveals that PLK2 siRNA transfected HUVECs display reduced lamellipodia when compared to control siRNA transfected HUVECs. Scale bar, 20 µm. Immunostaining of (C–C′′′, D–D′′′) pFAK and (E–E′′′, F–F′′′) integrin αVβ3 shows that focal adhesions and integrins, respectively, are localized to the lamellipodia of migrating/extending (C and E) control siRNA transfected HUVECs, but they fail to organize and aggregate in (D and F) PLK2 siRNA transfected HUVECs. Scale bar, 10 µm. (G) Quantitative measurements of the number of lamellipodia and filopodia reveal that zebrafish plk2b knockdowns have reduced lamellipodia but relatively the same number of filopodia (finger-like protrusions crossing the cell edge) when compared to controls. Conversely, zebrafish RNA injection of 160 pg plk2b resulted in more lamellipodia only. (H) Quantitative measurements of the number of lamellipodia and filopodia reveal that human PLK2 knockdowns have reduced lamellipodia (p=0.0023) but relatively the same number of filopodia (p=0.2615) when compared to controls. (I) Quantitative measurements of the number of pFAK (p=0.0143) and integrin αVβ3 plaques (p=0.0224) reveals that knockdown of PLK2 reduced the number of cell adhesions in HUVECs. (J) EC adhesion assays show that PLK2 siRNA HUVECs adhered less to type I collagen (p=0.0017) and fibronectin-coated coverslips (p=0.0061) compared to control siRNA HUVECs. Arrowheads and arrows point to lamellipodia and filopodia, respectively. Red – phalloidin/actin staining; Blue – DAPI staining; Green – (A) GFP, (C–D) pFAK, or (E and F) integrin αVβ3. Mean±s.e.m. *p<0.05, **p<0.01 by ANOVA for G and Student′s t-test for H–J. |
|
VEGF and Notch regulate PLK2 expression in HUVECs and zebrafish vasculature. (A)RT-PCR and (B) Western analysis reveals that PLK2 mRNA and protein expression levels were elevated in HUVECs after VEGF treatment. (C) Quantitative measurements show that PLK2 mRNA (p=0.004) and protein level (p=0.015) in HUVECs is less after VEGF treatment. (D–G) Whole mount RNA in situ hybridization shows that (E, E′) VEGF pathway inhibition with SU5416 (n=29/33) treatment starting at 20 hpf resulted in decreased plk2b expression in the zebrafish vasculature at 32 hpf when compared to (D, D′) DMSO treatment (n=0/35), whereas (G, G′) Notch inhibition with dn-MAML overexpression (n=24/35) starting at 20 hpf resulted in increased plk2b expression in the zebrafish vasculature at 32 hpf when compared to the (F, F′) control siblings (n=0/35). D′–G′ images represent the boxed area in D–G. (H, I) Quantitative PCR confirms that plk2b expression level in sorted endothelial cells is decreased in (H) SU5416 treated fish (p=0.0178) and (I) increased in dn-MAML overexpression fish (p=0.0142). Mean±s.e.m. *p<0.05, **p<0.01 by Student′s t-test. |
|
plk2b sense control in situ expression analysis reveals that plk2b expression in the vasculature is specific. (A–D) plk2b sense probe control in situ hybridization study shows that plk2b expression in the embryonic zebrafish vascular system is specific. No plk2b sense probe can be observed at 16 hpf (n=20), 24 hpf (n=23), 36 hpf (n=27) and 48 hpf (n=24) in the vasculature. |
|
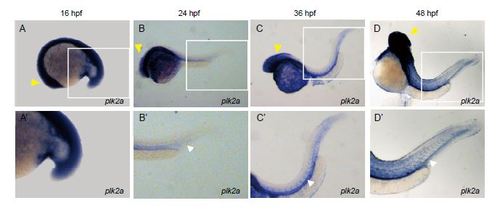
plk2a is expressed more broadly in the zebrafish brain/nervous system and weakly in the vasculature. (A–D) Whole mount RNA in situ hybridization shows that plk2a is expressed more broadly in the zebrafish brain and nervous system (yellow arrowheads) and weakly in the vasculature (white arrowheads) at 16 hpf (n=23/27), 24 hpf (n=37/38), 36 hpf (n=41/45) and 48 hpf (n=39/43). A′–D′ are enlarged images of the boxed area of A–D. EXPRESSION / LABELING:
|
|
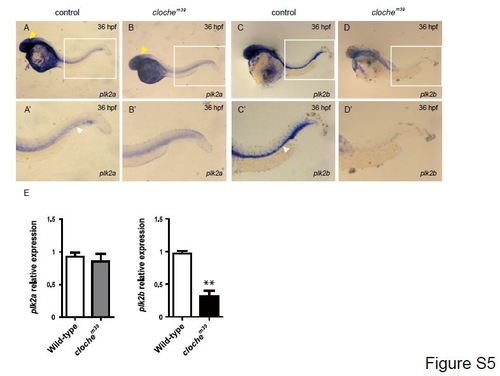
plk2a and plk2b expression in cloche mutant embryos. (A, C) Whole mount RNA in situ hybridization in wild-type sibling clochem39 control embryos shows that (A) plk2a is expressed broadly in the brain and nervous system (yellow arrowheads) and weakly in the vasculature (white arrowheads) (n=21/21), whereas (C) plk2b is expressed primarily in the vasculature (n=19/19) at 36 hpf. (B, D) However, in clochem39 mutants, which lack endothelial cells, plk2b (n=12/12) and plk2a (n=10/11) expression is reduced in the vasculature, but plk2a appears to be still expressed in the brain and nervous system at 36 hpf. A′–D′ are enlarged images of the boxed area of A–D. (E) Quantitative PCR shows that plk2b (p=0.003) but not plk2a (p=0.4096) expression is redcued in the clochem39 when compared to the wild-type siblings. Mean±s.e.m. **p<0.01 by Student′s t-test. |
|
Loss of plk2b function causes endothelial cell sprouting defects. (A) The following regions of plk2b were targeted to create the plk2b ATG (MO1) and splice (MO2) morpholinos (MO). The blue line indicates the 5′UTR, introns and 3′UTR. The green boxes indicate the exons. (B–G) plk2b MO1 blocks GFP expression from a construct in which the target sequence is placed upstream of the GFP coding sequence (plk2b-ATG-GFP). (B, C) RNA injected from the plk2b-ATG-GFP construct results in GFP expression throughout the embryo (n=31/31 GFP+). (D, E) Injection of the plk2b MO1 reduced the green signal of the fused plk2b ATG-GFP RNA (n=0/44 GFP+). (F, G) Injection of the plk2b mismatch control MO failed to reduce the green signal of fused plk2b ATG-GFP RNA (n=41/41 GFP+). (B, D, F) GFP fluorescence microscopy. (C, E, G) Brightfield microscopy. Scale bar – 1.5 mm. (H) Gel electrophoresis image shows that the plk2b splice MO2 blocks the proper splicing of plk2b and results in a larger 750 bp amplicon because of the inclusion of intron 2. Sanger sequencing of this amplicon showed an in-frame stop codon at the beginning of intron 2, which would block the translation of the rest exons of plk2b mRNA. Lane 1 – 100 bp ladder, Lane 2 – control MO, Lane 3 – MO2. (I) Loss of Plk2b function (plk2b MO1, plk2b MO2, dn-plk2b mRNA, BI 2536 treatment) results in an increased percentage of embryos with ISV defect. However, co-injection with zebrafish plk2b RNA (plk2b MO1+80 pg plk2b and plk2b MO2+80 pg plk2b), human PLK2 RNA (plk2b MO1+hPLK2), or ca-RAP1 RNA (plk2b MO1+100 pg ca-RAP1) can rescue this vascular defect as seen by the reduced percentage of ISV defects in plk2b MO embryos. Although injecting 80 pg of plk2b RNA did not result in ISV defects, injecting 160 pg of plk2b or 80 pg of ca-RAP1 RNA results in an increased percentage of embryos with ISV branching defects. n indicates the number of embryos with vascular phenotype/total number of embryos injected or treated for each condition. (J, K) Treating Tg(kdrl:GFP) fish with the PLK inhibitor, BI 2536, at 20 hpf decreased endothelial cell sprouting at 48 hpf (n=77/91) when compared to DMSO treated fish (n=0/43). Top, dorsal longitudinal anastomotic vessel; bottom, dorsal aorta/cardinal vein. Scale bar – 80 µm. |
|
plk2b morphants develop normally without apparent developmental delays. (A-C′′) Fluorescence and bright-field micrographs show that injection of (B–B′′′) plk2b MO1 and (C–C′′′) plk2b MO2 in Tg(kdrl:mcherry-ras) zebrafish embryos results in underdeveloped intersomitic vessel sprouting (open arrowheads), without general morphological abnormalities in whole embryo development at 48 hpf. (A–A′) control MO (n=76/76); (B–B′) plk2b ATG MO (MO1) (n=51/83); (C–C′) plk2b splice MO (MO2) (n=35/87). Scale bar, 500 µm. A′′–A′′′, B′′-B′′′, C′′–C′′′ panels are enlarged images of the boxed area of A–C, A′–C′, respectively. Scale bar, 200 µm. Top, dorsal longitudinal anastomotic vessel (yellow asterisk); bottom, dorsal aorta/cardinal vein. |
|
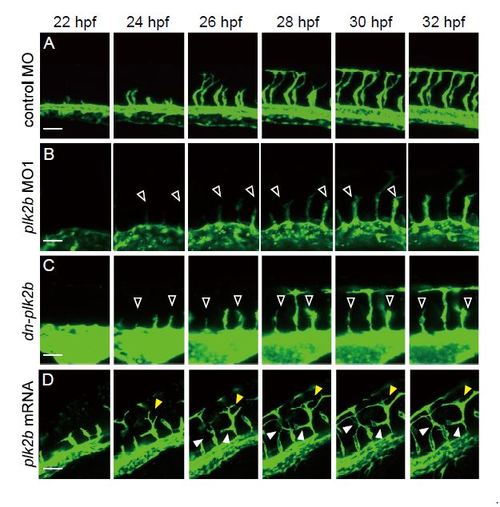
plk2b regulates intersomitic vessel angiogenic sprouting during zebrafish vascular development. (A–D) Time-lapse imaging of EC migration during intersomitic vessel (ISV) elongation in Tg(fli1a:eGFP) fish from 22–32 hpf shows that ISVs of (B) plk2b MO1 injected and (C) dn-plk2b RNA injected fish failed to sprout and elongate to form the DLAV (open arrowheads), whereas (D) 160 pg plk2b RNA injected fish appeared to not only develop the DLAV earlier (yellow arrowheads), but also grow additional ISV branches that connect throughout the somites (white arrowheads). Top, dorsal longitudinal anastomotic vessel; bottom, dorsal aorta/cardinal vein. Scale bar, 80 µm. |
|
plk2b regulates zebrafish endothelial cell migration and sprouting in vivo via Rap1 activity. (A) Injecting 100 pg of ca-RAP1 into (A) plk2b MO Tg(kdrl:mcherry-ras) fish could partially rescue the plk2b MO vascular defects (n=42/127). (B) Additionally, injecting 160 pg of ca-RAP1 into plk2b MO Tg(kdrl:mcherry-ras) fish could not only partially rescue the plk2b MO vascular defects but also occasionally lead to ectopic EC branching (yellow arrowheads) (n=41/78). (C) Injecting 80 pg of ca-RAP1 into wild-type Tg(kdrl:mcherry-ras) fish also resulted in occasional ectopic EC branching (yellow arrowheads) (n=57/127). Scale bar, 80 µm. Top, dorsal longitudinal anastomotic vessel; bottom, dorsal aorta/cardinal vein. (D) Quantitative measurements of the number of ISV branches show more ISV branches in 80 pg ca-RAP1 injected embryos and in plk2b MO1 embryos rescued with 160 pg ca-RAP1 when compared to control and plk2b MO1 embryos rescued with 100 pg ca-RAP1. Mean±s.e.m. *p<0.05 by ANOVA. |
Reprinted from Developmental Biology, 404(2), Yang, H., Fang, L., Zhan, R., Hegarty, J.M., Ren, J., Hsiai, T.K., Gleeson, J.G., Miller, Y.I., Trejo, J., Chi, N.C., Polo-like kinase 2 regulates angiogenic sprouting and blood vessel development, 49-60, Copyright (2015) with permission from Elsevier. Full text @ Dev. Biol.