- Title
-
Otolith tethering in the zebrafish otic vesicle requires Otogelin and α-Tectorin
- Authors
- Stooke-Vaughan, G.A., Obholzer, N.D., Baxendale, S., Megason, S.G., Whitfield, T.T.
- Source
- Full text @ Development
|
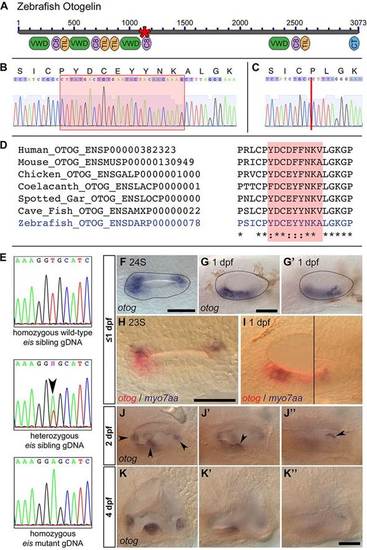
Development of otoliths, hair cells and cilia in eis mutant zebrafish embryos. (A-D) Live DIC images; lateral views with anterior to the left and dorsal up. (A) Phenotypically wild-type 26S sibling OV containing two tethered otoliths (arrowheads). (A′) Magnified view of the anterior OV pole from A. (B) 26S eis mutant OV. Otolith seeding has failed and there is an accumulation of OPPs in the OV. (B′) Magnified view of the anterior OV pole from B. (C) 26hpf sibling OV. The otoliths (arrowheads) have started to biomineralise. (D) 26hpf eis mutant OV. A single, large biomineralised otolith (arrowhead) has formed and is tethered above the posterior macula. (E,F) Maximum projection of confocal stacks through the entire OV of a 25S wild-type (AB strain) embryo (E) and <25S eis mutant embryo (F) stained with anti-acetylated Tubulin antibody. Dorsal view, with anterior left, lateral down. Dashed line indicates the approximate location of the OV lumen. There is no obvious difference in ciliary morphology in the eis mutant ear. (G) myo7aa mRNA expression marks tether cell pairs at the OV poles in a 28S phenotypically wild-type sibling. Dorsal view, with anterior to left. (H) myo7aa expression is unaffected in 28S eis mutant embryos. (I,J) Time-to-colour merge of six consecutive frames from movies of a 24hpf wild-type (AB strain) OV (I; supplementary material Movie 1) and a 25hpf eis mutant OV (J; supplementary material Movie 2). Dorsolateral views of left OVs are shown with anterior to right. Colour indicates movement, greyscale indicates lack of movement. Black arrowheads mark immotile kinocilia, white arrowheads mark motile cilia. (K) Tapping of eis mutant embryos showed that the single otolith tethers at 27-28hpf (n=3 for each time point). (L) Fluorescent hair cell counts in Tg(pou4f3:mgfp) embryos indicate that a second wave of hair cells differentiates from 27hpf. n=3 ears for 24.5 hpf; n=4 ears for all other ages. Error bars indicate s.e.m. Scale bars: 40µm in A-D; 10µm in A′,B&prime& 40µm in E,F; 50µm in G,H; 10µm in I,J. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The eis mutation corresponds to a lesion in otog. (A) Overview of Otogelin protein structure based on available sequence data; the N terminus is likely to be incomplete. The asterisk indicates the predicted in-frame deletion in the eiste296f allele. VWD, Von Willebrand factor type D domain; C8, domain containing eight conserved cysteine residues; TIL, trypsin inhibitor-like cysteine-rich domain; CT, C-terminal cysteine knot-like domain. (B) Pooled wild-type (strain LWT) cDNA sequence data and predicted amino acid translation covering the region of otog that is mutated in eiste296f. The red box indicates the sequence deleted in eiste296f. (C) Pooled eiste296f cDNA from the same region as shown in B. The deletion is indicated by a vertical red line. (D) Clustal 2.1 multiple sequence alignment of the region of Otogelin deleted in the eiste296f allele. The nine amino acids deleted in eiste296f are highly conserved across vertebrates (shading). (E) gDNA sequence data from wild-type sibling, eiste296f heterozygous sibling and homozygous mutant embryos, confirming the T-to-A transversion identified by HMFseq (arrowhead indicates the double peak in the heterozygous embryo). (F-G2) Dorsal (F, 24S) and lateral (G,G2, 1dpf) views of a wild-type OV (dotted outline), showing that otog mRNA expression is not restricted to the tether cells. (G,G′) Different focal planes of the same OV. Anterior is to left. (H) Dorsal view of 23S wild-type OV; anterior to left. Expression of otog mRNA (red) includes the tether cells (marked by myo7aa, blue) and other cells at the poles of the OV. (I) Lateral view of 1dpf wild-type OV. Two focal planes are combined (black line marks the join), showing the anterior macula (left) and the posterior macula (right). otog (red) is expressed in hair cells (myo7aa positive, blue) and surrounding epithelial cells. (J-J3) Lateral view of different focal planes of the same 2dpf wild-type OV, showing expression of otog in the cristae (J, arrowheads), at the posterior of the anterior macula (J′, arrowhead) and along the dorsal edge of the posterior macula (J′′, arrowhead). (K-K′′) At 4dpf, otog is still expressed in the cristae, but expression is now very weak in the maculae (the apparent macula stain in K2 is out-of-focus staining in the lateral crista). Scale bars: 50µm. EXPRESSION / LABELING:
|
|
Development of otoliths in rst mutant and rst;eis double-mutant embryos. Live DIC micrographs of sibling and rst mutant OVs. The left OV is shown, with anterior to the left and dorsal to the top. (A) 25S embryo from a homozygous mutant rst cross. The otoliths seed normally at the anterior and posterior poles (compare with Fig. 1A). (B) 5dpf phenotypically wild-type sibling OV. p, posterior otolith. (C) 5dpf rst mutant OV. The posterior otolith is misshapen, detached and free to move. (D) OV of a different rst mutant, with a posterior otolith that has become detached and stuck on the ventral floor of the OV. (E) OV of an rst mutant with the most common phenotype of a misshapen posterior otolith. (F) 5dpf eis mutant with a single otolith tethered to the posterior macula. (G) 5dpf rst mutant with an untethered posterior otolith. (H) 5dpf rst;eis double mutant with a single untethered otolith. (H′) The same embryo as in H, after the slide was tapped to displace the untethered otolith. Numbers indicate embryos showing each phenotype (detached or misshapen posterior otolith) among total examined (130) from an rst;eis double-heterozygote cross; the remaining 76/130 embryos were phenotypically wild type (not shown). Scale bars: 50µm in A; 100µm in B-H′. PHENOTYPE:
|
|
The rst mutation corresponds to a lesion in tecta. (A) Overview of α-Tectorin protein structure based on available sequence data; the N terminus is likely to be incomplete. The asterisk shows the location of the predicted truncation in the rsttl20e allele. NIDO, nidogen domain; VWD, Von Willebrand factor type D domain; C8, domain containing eight conserved cysteine residues; TIL, trypsin inhibitor-like cysteine-rich domain; ZP, zona pellucida domain. (B) Sequencing data from rst mutant, heterozygous sibling and homozygous wild-type sibling embryos showing the T-to-A transversion (arrowhead and asterisk). (C) tecta mRNA expression in the OV of a 1dpf phenotypically wild-type sibling embryo from an eis cross. tecta is expressed in the utricular (U) and saccular (S) maculae. (D) 2dpf wild-type (AB strain) embryo showing that tecta is expressed exclusively in the OV (arrowhead). (E,E2) Within the OV at 2dpf, tecta is expressed at high levels in the utricle and saccule, with lower levels in the semicircular canal projections (black arrowhead) and at the base of the cristae (white arrowheads). (F,F2) Strong tecta expression continues in the utricular and saccular maculae at 3dpf. (G,G2) The level of tecta expression in the maculae is reduced at 5dpf, and a new region of expression appears (arrowhead). (H) Dorsal view of utricle (U) and saccule (S) in 4dpf wild-type embryo. L, lateral; P, posterior. Expression in the utricular macula is strongest at the periphery. (I,J) rst mutants show a lower level of tecta expression than siblings at 30hpf. Genotypes were confirmed by sequencing gDNA. (K) Dorsal view of a 23S wild-type OV, showing that tecta mRNA expression (red) is not restricted to the tether cells (myo7aa mRNA, blue) at seeding stages. (L,L′) Lateral views of 25hpf phenotypically wild-type OV. The focal plane shows the region of the anterior macula in L and the region of the posterior macula in L′. tecta expression includes the tether cells and surrounding cells of the anterior macula (L) and a region just anterior to the tether cells of the posterior macula (arrowhead, L′). (M) Dorsal view of a 23S wild-type OV, showing that otog (red) and tecta (blue) expression roughly overlap at this stage. (N,N′) Lateral views of a 25hpf phenotypically wild-type OV. The focal plane shows the region of the anterior macula in N and the region of the posterior macula in N′. otog (red) is expressed in a tecta-negative area of the ventral OV epithelium (arrowhead, N). Scale bar: 50µm in C,I,J,K-N&prime& 500µm in D; 33µm in H; 75µm in E-G′. EXPRESSION / LABELING:
|
|
Normal macular development is required for normal expression of otog and tecta. (A) otog mRNA expression at the poles of the OV of a 21hpf wild-type (LWT strain) OV. (B) Expression of otog was reduced in the OV of atoh1b morphants. (C) Expression of tecta at the poles of a 21hpf wild-type OV. (D) tecta expression was not detected in the OV of atoh1b morphants. (E) otog expression in the utricular macula of a 31hpf phenotypically wild-type sibling embryo. (F) otog expression was reduced in the utricular macula of a 31hpf mib mutant embryo. (G) tecta expression in the utricular macula of a 31hpf phenotypically wild-type sibling embryo. (H) tecta expression was reduced in the utricular macula of a 31hpf mib mutant embryo. Weak expression remained in the saccular macula (out of focus). Dorsal (A-D) and lateral (E-H) views, with anterior to left. Scale bar: 50µm for A-H. EXPRESSION / LABELING:
PHENOTYPE:
|
|
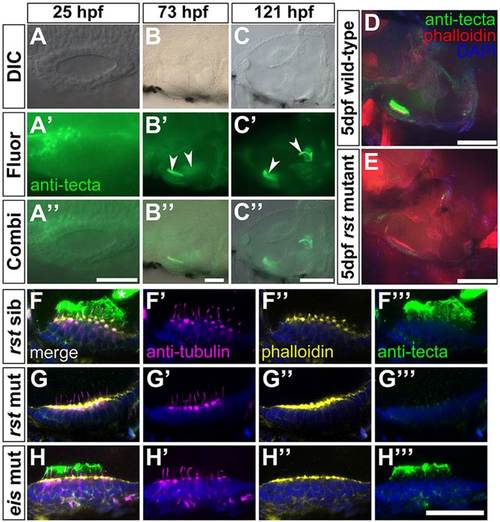
Expression of α-Tectorin protein in wild-type and rst mutant embryos. (A-C′′) Immunofluorescence analysis showing that α-Tectorin protein is localised to the anterior OV at 1dpf and to the two otolithic membranes at 3 and 5dpf (arrowheads). Anterior is to the left. (D) Confocal image showing α-Tectorin protein localisation to the utricular otolithic membrane and to cells in the utricular epithelium (green) in a 5dpf wild-type (AB strain) embryo. Anterior left, dorsal up. (E) In a 5dpf rst embryo, α-Tectorin is not localised to the otolithic membrane of the utricular macula, although there is a low level of protein detectable within the utricular epithelium. (F-H′′′) Confocal images of utricular maculae from 5dpf embryos; lateral views, anterior to left. Nuclei are stained with DAPI (blue), hair cells and kinocilia with anti-acetylated Tubulin antibody (magenta), filamentous actin and hair cell stereociliary bundles with Alexa647-phalloidin (yellow), and α-Tectorin by antibody (green). (F-F′′′) Phenotypically wild-type 5dpf rst sibling embryo, showing strong staining for α-Tectorin in the utricular otolithic membrane, and protrusion of the hair cell kinocilia into this membrane. α-Tectorin staining is also visible in the saccular otolithic membrane (asterisk, F). (G-G′′′) 5dpf rst mutant embryo with no extracellular α-Tectorin stain. There is a weak α-Tectorin signal at the apical surface of the hair cells (G′′′). Kinocilia appear normal (G′). (H-H′′′) 5dpf eis mutant embryo with normal expression and localisation of α-Tectorin and normal kinocilia. Scale bars: 50µm in A-B′&prime& 100µm in C-E; 20µm in F-H′′′. |