- Title
-
Mitochondrial Ca(2+) uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity
- Authors
- Shimizu, H., Schredelseker, J., Huang, J., Lu, K., Naghdi, S., Lu, F., Franklin, S., Fiji, H.D., Wang, K., Zhu, H., Tian, C., Lin, B., Nakano, H., Ehrlich, A., Nakai, J., Stieg, A.Z., Gimzewski, J.K., Nakano, A., Goldhaber, J.I., Vondriska, T.M., Hajnóczky, G., Kwon, O., Chen, J.N.
- Source
- Full text @ Elife
|
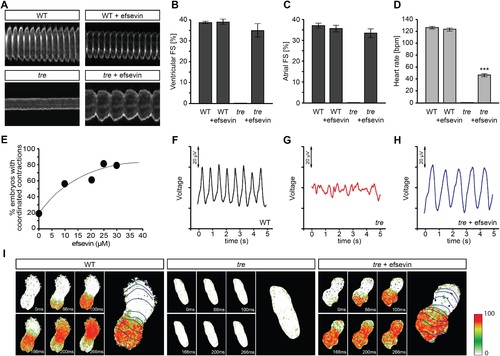
Efsevin restores rhythmic cardiac contractions in zebrafish tremblor embryos. (A) Line scans across the atria of Tg(myl7:GFP) embryonic hearts at 48 hpf. Rhythmically alternating systoles and diastoles are recorded from vehicle- (upper left) or efsevin- treated wild type (upper right) and efsevin-treated tre (lower right) embryos, while only sporadic unsynchronized contractions are recorded from vehicle-treated tre embryos (lower left). (B, C) Fractional shortening (FS) deduced from the line-scan traces. While cardiac contraction was not observed in tre, efsevin-treated wild type and tre hearts have similar levels of FS to those observed in control hearts. Ventricular FS of wild type v.s. wild type + efsevin vs tre + efsevin: 39 ± 0.6%, n = 8 vs 39 ± 1%, n = 10 vs 35 ± 3%, n = 6; and Atrial FS: 37 ± 1%, n = 11 vs 35 ± 2%, n = 11 vs 33 ± 2%, n = 15. (D) While efsevin restored a heart rate of 46 ± 2 beats per minute (bpm) in tre embryos, same treatment does not affect the heart rate in wild type embryos (126 ± 2 bpm in vehicle-treated embryos vs 123 ± 3 bpm in efsevin-treated wild-type embryos). ***, p < 0.001 by one-way ANOVA. (E) Dose-dependence curve for efsevin. The tre embryos were treated with various concentrations of efsevin from 24 hpf and cardiac contractions were analyzed at 48 hpf. (F-H) Representative time traces of local field potentials for wild type (F), tre (G) and efsevin-treated tre (H) embryos clearly display periods of regular, irregular, and restored periodic electrical activity. (I) In vivo optical maps of Ca2+ activation represented by isochronal lines every 33 ms recorded from 36 hpf wild type (left), tre (center) and efsevin-treated tre (right) embryos. PHENOTYPE:
|
|
(A) In situ hybridization analysis showed that VDAC2 is expressed in embryonic hearts at 36 hpf (upper image) and 48 hpf (lower image). (B) Injection of 25 pg in vitro synthesized VDAC2 mRNA restored cardiac contractions in 52.9 ± 12.1% (n = 78) of 1-day-old tre embryos, compared to 21.8 ± 5.1% in uninjected siblings (n = 111). (C) Schematic diagram of myl7:VDAC2 construct (top). In situ hybridization analysis showed that TBF treatment induces VDAC2 expression in the heart (lower panel). (D) While only ~20% of myl7:VDAC2;NCX1hMO embryos have coordinated contractions (n = 116), 52.3 ± 2.4% of these embryos established persistent, rhythmic contractions after TBF induction of VDAC2 (n = 154). (E) On average, 71.2 ± 8.8% efsevin treated embryos have coordinated cardiac contractions (n = 131). Morpholino antisense oligonucleotide knockdown of VDAC2 (MOVDAC2) attenuates the ability of efsevin to suppress cardiac fibrillation in tre embryos (45.3 ± 7.4% embryos with coordinated contractions, n = 94). (F) Efsevin treatment restores coordinated cardiac contractions in 76.2 ± 8.7% NCX1MO embryos, only 54.1 ± 3.6% VDAC2zfn/zfn;NCX1MO embryos have coordinated contractions (n = 250). (G) Diagram of Zinc finger target sites. VDAC2zfn/zfn carries a 34 bp deletion in exon 3 which results in a premature stop codon (red asterisk). In situ hybridization analysis showing loss of VDAC2 transcripts in VDAC2zfn/zfn embryos. White arrowheads point to the developing heart. |
|
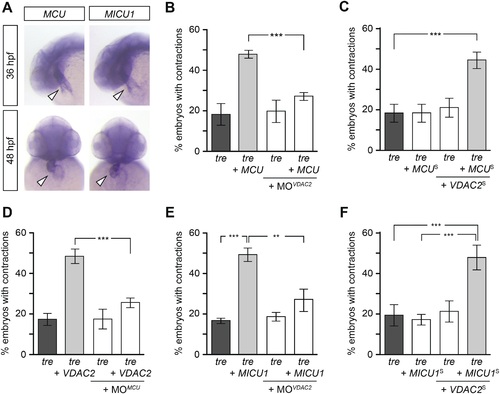
Mitochondria regulate cardiac rhythmicity through a VDAC2-dependent mechanism.(A) MCU and MICU1 are expressed in the developing zebrafish hearts (arrowhead). (B) Overexpression of MCU is sufficient to restore coordinated cardiac contractions in tre embryos (47.1 ± 1.6% embryos, n = 112 as opposed to 18.3 ± 5.3% of uninjected siblings, n = 64) while this effect is significantly attenuated when co-injected with morpholino antisense oligonucleotide targeted to VDAC2 (27.1 ± 1.9% embryos, n = 135). (C) Suboptimal overexpression of MCU (MCUS) and VDAC2 (VDAC2S) in combination is able to suppress cardiac fibrillation in tre embryos (42.9 ± 2.6% embryos, n = 129). (D) The ability of VDAC2 to restore rhythmic contractions in tre embryos (48.5 ± 3.5% embryos, n = 111) is significantly attenuated when MCU is knocked down by antisense oligonucleotide (MOMCU) (25.6 ± 2.4% embryos, n = 115). (E) Overexpression of MICU1 is sufficient to restore rhythmic cardiac contractions in tre embryos (49.3 ± 3.4% embryos, n = 127 compared to 16.8 ± 1.4% of uninjected siblings, n = 150). This effect is abrogated by VDAC2 knockdown (MOVDAC2, 25.3 ± 5.5% embryos, n = 97). (F) Suboptimal overexpression of MICU1 (MICU1S) and VDAC2 (VDAC2S) in combination is able to restore rhythmic cardiac contractions in tre embryos (48.6 ± 6.0%, n = 106). Error bars represent s.d.; *p < 0.05; ***p < 0.001. |