- Title
-
The Rac1 regulator ELMO controls basal body migration and docking in multiciliated cells through interaction with Ezrin
- Authors
- Epting, D., Slanchev, K., Boehlke, C., Hoff, S., Loges, N.T., Yasunaga, T., Indorf, L., Nestel, S., Lienkamp, S.S., Omran, H., Kuehn, E.W., Ronneberger, O., Walz, G., Kramer-Zucker, A.
- Source
- Full text @ Development
|
ELMO1 and DOCK1 show expression in cilia in human and zebrafish and are required for ciliogenesis in zebrafish. (A-E) Human respiratory epithelial cells from healthy controls were double labelled with antibodies against ELMO1 (green) and CEP164 (magenta). ELMO1 localises along the ciliary axonemes (arrow) and the basal bodies (arrowhead). The nucleus is stained with Hoechst 33342 (blue). (F) Transverse section of a 24 hpf zebrafish embryo reveals that elmo1 mRNA expression (blue) partially colocalises with cadherin 17 (cdh17) mRNA (red) in the pronephric tubule. (G-I) Dock1 is expressed at the base of the cilium (arrowheads) and ciliary axonemes (green fluorescence of Arl13b-GFP) in 48 hpf Tg(actb2:Mmu.Arl13b-GFP) zebrafish embryos. (J-K′′) Expression silencing of elmo1 (J-J′′) and dock1 (K-K′′) using SB-MO elmo1 (2 ng) and SB-MO dock1 (2 ng), respectively, resulted in pronephric cyst formation (arrowheads and stars), as shown in a bright-field lateral view with anterior to the left (J,K), a dorsal view with anterior to the left of a Tg(wt1b:EGFP) embryo (J′,K′), and in a transverse section (J′′,K′′) of 48 hpf embryos. (L) Quantification of pronephric cyst formation in 48 hpf zebrafish embryos injected with Co-MO, SB-MO dock1, TB-MO elmo1 and SB-MO elmo1 (each 2 ng) or SB-MO elmo1 (2 ng) + elmo1 mRNA (20 pg). There was significant prevention of cyst formation upon co-injection of elmo1 mRNA (*P <= 0.05). The number of individual embryos analysed is indicated above each bar. (M-O′) Analysis of electron micrographs revealed basal body docking defects (arrows) in 48 hpf zebrafish embryos injected with SB-MO elmo1 (2 ng) (N,N′) or SB-MO dock1 (2 ng) (O,O′) as compared with Co-MO (2 ng) (M,M′). Scale bars: 10 μm in D; 20 μm in F,J′,K′ ; 100 μm in I; 50 μm in J,K,J′′,K′′ ; 2 μm in M,N,O ; 0.5 μm in M′,N′,O′. EXPRESSION / LABELING:
PHENOTYPE:
|
|
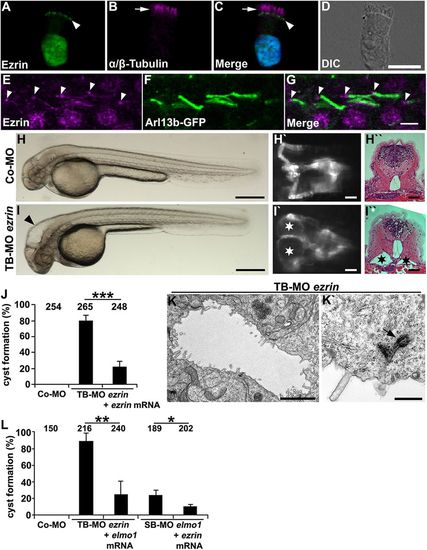
Ezrin shows expression in cilia and is required for ciliogenesis in zebrafish. (A-D) Human respiratory epithelial cells from healthy controls were double labelled with antibodies directed against Ezrin (green) and the ciliary axoneme marker α / β -Tubulin (magenta) (arrow). Ezrin localisation is restricted to the putative basal bodies (arrowhead) and the nucleus. The nucleus is stained with Hoechst 33342 (blue). (E-G) Ezrin is expressed at the basal bodies (arrowheads) and the ciliary axonemes in 48 hpf Tg(actb2:Mmu.Arl13b-GFP) zebrafish embryos. (H-I′′) Expression silencing of ezrin (I-I′′) using TB-MO ezrin (2 ng) results in hydrocephalus (arrowhead in I) and pronephric cyst formation (stars in I′ and I′′) as compared with zebrafish embryos injected with Co-MO (2 ng) (H-H′′), shown in a bright-field lateral view with anterior to the left (H,I), a dorsal view with anterior to the left of a Tg(wt1b:EGFP) embryo (H′,I′), and by a histological transverse section (H′′,I′′) of 48 hpf embryos. (J) Quantification of pronephric cyst formation in 48 hpf zebrafish embryos after injection with TB-MO ezrin (2 ng) or TB-MO ezrin (2 ng) + ezrin mRNA (20 pg), as compared with Co-MO (2 ng). There is significant prevention of cyst formation upon co-injection of ezrin mRNA (***P<0.001). (K,K′) TEM analysis revealed reduced microvilli formation and basal body docking defects in TB-MO ezrin (2 ng) morphants at 48 hpf. Arrow indicates prospective basal body not properly docked. (L) Quantification of pronephric cyst formation in 48 hpf zebrafish embryos injected with Co-MO (2 ng), TB-MO ezrin (2 ng), TB-MO ezrin (2 ng) + elmo1 mRNA (20 pg), SB-MO elmo1 (2 ng) or SB-MO elmo1 (2 ng) + ezrin mRNA (20 pg) (*P=0.03; **P=0.006). (J,L) The number of individual embryos analysed is indicated above each bar. Scale bars: 10 μm in D; 5 μm in G; 100 μm in H,I; 50 μm in H′,H′′,I′,I′′ ; 2 μm in K; 0.5 μm in K′. |

Rac1 is required for ciliogenesis in zebrafish and Xenopus. (A-B′′) Expression silencing of Rac1l using TB-MO rac1l (0.5 ng) results in pronephric cyst formation (stars in B′ and B′′) as compared with Co-MO (0.5ng) morphants (A′), as shown in a dorsal view with anterior to the left of a Tg(wt1b:EGFP) zebrafish embryo (A′,B′) and in a histological transverse section (B") of a 48 hpf embryo. (A,B) Embryos are shown in bright-field lateral view, with anterior to the left. (C) Quantification of pronephric cyst formation in 48 hpf zebrafish embryos injected with Co-MO (4.5 ng), TB-MO rac1 (4 ng), TB-MO rac1l (0.5 ng), TB-MO rac1 (4 ng)/rac1l (0.5 ng) and TB-MO rac1l (0.5 ng)+ rac1l mRNA (20pg). There was significant prevention of cyst formation by co-injection with rac1l mRNA (*Pd0.05). (D) Quantification of laterality defects by cmlc2 in situ hybridisation revealed impaired heart looping in 48 hpf zebrafish embryos injected with TB-MO rac1 (4ng), TB-MO rac1l (0.5 ng), and TB-MO rac1 (4 ng)/rac1l (0.5 ng) compared with Co-MO (4.5 ng). (C,D) The number of individual embryos analysed is indicated above each bar. (E-E′′) Analysis of electron micrographs revealed basal body docking defects (arrows in E′,E′′) on sections of 48 hpf zebrafish embryos injected with TB-MO rac1 (4 ng)/rac1l (0.5 ng). (F-F′′′) Xenopus embryos injected with Co-MO (40 ng) and centrin-GFP mRNA showed normal basal body docking and basal body distribution at stage 32; embryos were colabelled with phalloidin 568 (magenta). (G-H′′′) Xenopus embryos (stage 32) injected with TB-MO rac1 (40 ng) exhibited irregular basal body docking and distribution, ‘beads on a string’ pattern in H. Scale bars: 100 μm in A,B; 20 μm in A′,B′ ; 50 μm in B′′ ; 1 μm in E-E′′ ; 5 μm in H′′′. |
|
The ELMO1-DOCK1-Rac1 complex influences ERM phosphorylation and tight control of phospho-Ezrin is required for proper ciliogenesis. (A) Immunoblot of 24 hpf zebrafish lysates showing elevated phospho-Ezrin (pERM) levels in embryos injected with TB-MO rac1 (4ng)/rac1l (0.5ng), SB-MO elmo1 (2ng) and SB-MO dock1 (2ng) as compared with Co-MO (4.5ng). (B) Immunoblot of 24 hpf zebrafish lysates showing reduced pERM levels in zebrafish embryos injected with elmo1 mRNA (20pg) or rac1l mRNA (20pg) as compared with control embryos. Anti-γ-Tubulin immunoblots served as a loading control. Immunoblots represent results from one of three independent experiments with similar results. (C-F) Overexpression of Ezrin by transient transgenesis demonstrates the importance of tight regulation of phosphorylation at threonine 564 for the function of zebrafish Ezrin. cadherin 17 (cdh17) promoter-driven expression of either the nonphosphorylatable T564A mutation or the phosphorylation mimetic mutant T564D caused cyst formation on the side of the pronephros where the MCCs in the mid-portion of the pronephric tubule are most affected. Arrowheads indicate the tubular mid-portion, with the cells expressing mutant Ezrin on the side of the formed cyst. The number of individual embryos analysed is indicated above each bar. (G) Quantification of pronephric cyst formation of 48 hpf zebrafish embryos injected with SB-MO elmo1 (2 ng) with or without ezrin(T564A) mRNA (10 pg) (**P=0.006), SB-MO dock1 (2 ng) with or without ezrin(T564A) mRNA (10 pg) (**P=0.009) or TB-MO rac1 (4 ng)/rac1l (0.5 ng) with or without ezrin(T564A) mRNA (10 pg) (*P=0.02). Scale bars: 50 μm. EXPRESSION / LABELING:
|