- Title
-
FIH-1, a Novel Interactor of Mindbomb, Functions as an Essential Anti-Angiogenic Factor during Zebrafish Vascular Development
- Authors
- So, J.H., Kim, J.D., Yoo, K.W., Kim, H.T., Jung, S.H., Choi, J.H., Lee, M.S., Jin, S.W., Kim, C.H.
- Source
- Full text @ PLoS One
|
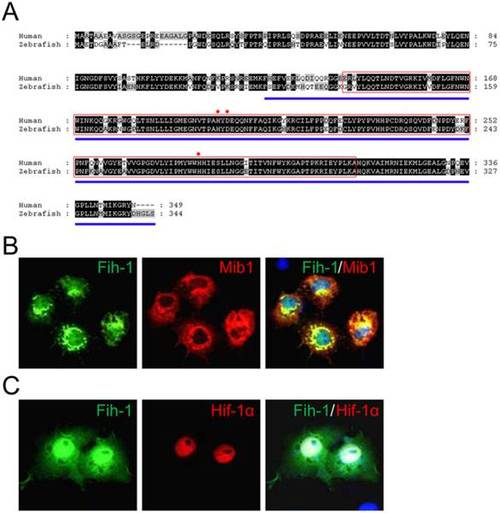
Association of Factor Inhibiting HIF-1α (FIH-1) with Mindbomb. (A) Amino acid sequence comparison between human and zebrafish FIH-1/Fih-1. JmjC domain is marked as a red box. Region of FIH-1 which mediates its interaction with HIF-1α is marked as blue underline. Red asterisks indicate metal coordination sites. (B) Zebrafish Fih-1 co-localizes with Mib1 in transiently transduced Cos7 cells. (C) Zebrafish Fih-1 co-localizes with Hif1α in transiently transduced Cos7 cells. Expression vectors encoding HA-Mib1 and Fih-1-GFP (For B) or HA-Hif-1α and Fih-1-GFP (For C) were used. |
|
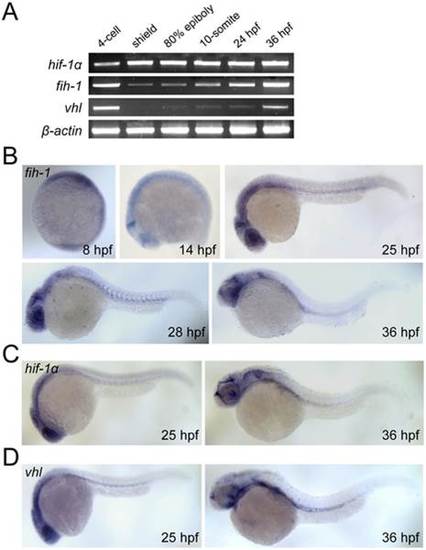
Expression pattern of fih-1, vhl, and hif-1α. (A) RT-PCR analysis showing expression of fih-1, hif-1a, vhl, and β-actin during development. (B) Whole-mount in situ hybridization of fih-1 during development. As early as 8 hpf, fih-1 expression can be detected. At 14 hpf, fih-1 is strongly expressed at the midbrain-hindbrain boundary and eye. At 25 hpf, expression of fih-1 is expanded to the optic vesicle and ventral mesoderm. The expression of fih-1 in midbrain-hindbrain boundary, eye, optic vesicle and ventral mesoderm is maintained at later stages. (C) Expression of hif1α, a known target of Fih-1, at 25 hpf and 36 hpf. (D) Expression of vhl, which is known to interact and synergize with Fih-1, at the same developmental stage. Both hif1α and vhl express within the similar anatomical region as fih-1. |
|
Fih-1 negatively modulates angiogenesis during development. (A) Validation of the morpholino (MO) targeting fih-1. Injection of fih-1 MO interferes with mRNA splicing, leading to the retention of an intron and premature translation termination. (B) Gross morphology of fih-1 MO-injected embryos in comparison with control MO-injected embryos. No obvious gross morphological defects were observed at 55 hpf. (C) At 50 hpf, injection of fih-1 MO did not cause any obvious increase in angiogenic sprouts, however, exuberant angiogenic sprouts emerge from the intersegmental vessels (ISVs) in fih-1 MO-injected embryos at 80 and 122 hpf. (D) Concomitant injection of fih-1 mRNA can rescue exuberant angiogenesis caused by fih-1 MO-injected embryos. On the right column, control (top, n = 12), fih-1 MO (middle, n = 20), or fih-1 MO and fih-1 mRNA (bottom, n = 20) injected embryos. Arrows point to ectopic angiogenic sprouts. Quantification of ectopic angiogenic sprouts per embryo is shown on the right. Asterisk notes statistical significance in the number of ISVs between fih-1 MO and fih-1 MO and fih-1 mRNA injected embryos. Error bars are standard deviation. |
|
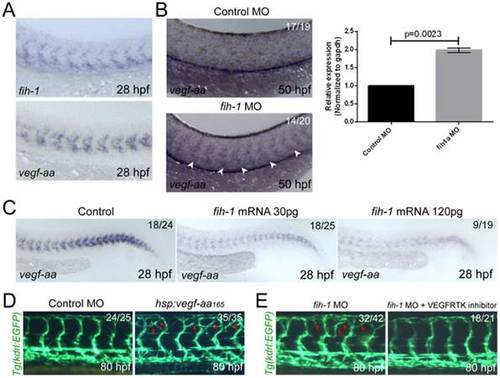
Fih-1 negatively regulates Vegf-aa165 in zebrafish embryos. (A) At 28 hpf, both fih-1 and vegf-aa165 transcript are selectively expressed within the anterior somites and ventral mesoderm. (B) Attenuation of Fih-1 activity in zebrafish elevates the level of vegf-aa165 expression at 50 hpf. Arrowheads point to vegf-aa165 expression in somites caused by lack of Fih-1 activity. Similarly, the level of vegfaa transcript in 48 hpf control or fih-1 MO-injected embryos was evaluated by quantitative RT-PCR. Error bars are standard deviation (right). (C) Ectopic expression of Fih-1 decreases the level of vegf-aa165 expression in a dose-dependent manner at 28 hpf. (D) Functional relationship between Fih-1 and Vegf-aa165. Ectopic expression of Vegf-aa165 under the regulation of hsp70l promoter caused a similar vascular phenotype as observed in fih-1 MO-injected embryos. Moreover, inhibition of Vegf-A signaling can drastically reduce the exuberant angiogenic sprouts in fih-1 MO-injected embryos at 80 hpf. Arrows point to ectopic sprouts. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Over-expression of Fih-1 inhibits the formation of angiogenic vessels during development. (A) Whole-mount in situ hybridization with fli1a and kdrl in control or fih-1 mRNA-injected embryos. In fih-1 mRNA-injected embryos, the number of intersegmental vessels (black arrowheads) was substantially reduced. (B) Microangiography of fih-1 mRNA-injected embryos shows a lack of circulation in intersegmental vessels. (C) Ectopic expression of vegf-aa165 restored impaired angiogenic vessel formation in fih-1 mRNA-injected embryos at 52 hpf. Arrowheads point to intersegmental vessels which fail to anastomose at the dorsal-most part of the embryos. (D) Schematic diagram of two constructs that encode either N-terminus or C-terminus deleted Fih-1. Both N-terminus only Fih-1 (Fih-1ΔC) and C-terminus only Fih-1 (Fih-1ΔN) contain JmjC domain and metal binding sites which are essential for hydroxylation of HIF-1α. (E) C-terminus of Fih-1 is essential for its anti-angiogenic activity. While full length fih-1 or fih-1ΔN mRNA-injected embryos inhibit angiogenic sprouts of intersegmental vessels, fih-1ΔC mRNA-injected embryos at 72 hpf do not show any obvious vascular defects. Arrowheads point to angiogenic sprouts which fail to extend to the dorsal-most side of the embryos. |
|
Specification of endothelial cells is not affected in fih-1 MO-injected embryos. Micrographs of whole-mount in situ hybridization with fli-1a (top rorw) and scl (bottom row) in control (left column) or fih-1 (right column) MO-injected embryos at 12 hpf. |
|
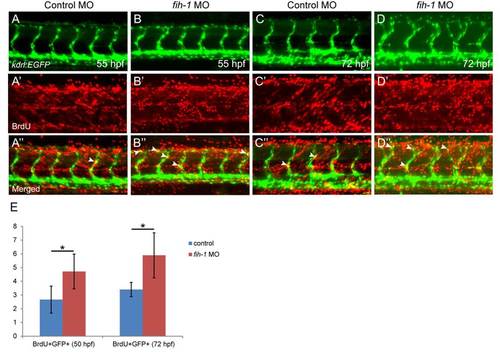
Fih-1 regulates endothelial cell proliferation. Proliferating endothelial cells in control (A and C) or fih-1 MO-injected (B and D) at 55 hpf (A and B) and 72 hpf (C and D). The number of BrdU positive endothelial cells within the intersegmental vessels (ISVs) was significantly increased in fih-1 MO-injected embryos at 55 and 72 hpf, compared to control embryos. Arrows indicate GFP+/BrdU+ endothelial cells in Tg(kdrl:EGFP) transgenic zebrafish (B′′ and D′′). Quantification on the number of GFP+/BrdU+ endothelial cells are shown in E. Asterisks indicate statistical significance (* p<0.005). Error bars, ±SD. n = 6 (55 hpf control), 5 (72 hpf control), 8 (55 hpf fih-1 MO-injected), and 9 (72 hpf fih-1 MO-injected).PHENOTYPE:
|
|
fih-1regulates hif-1a targets during zebrafish development. (A) Schematic diagram on negative regulation of Hif-1α by Fih-1. Whole-mount in situ hybridization of heme oygenase1a (hmox1a) (B) and glucose transporter-3 (glut3) (C) in control or fih-1 MO-injected embryos. Lack of Fih-1 strongly induces expression of known Hif-1α targets.EXPRESSION / LABELING:
|
|
Generation of Tg(hsp70l:vegfaa165)ck4. (A) Schematic diagram of the construct used to generate the Tg(hsp70l:vegfaa165) ck4 zebrafish line. 12 hpf Tg(hsp70l:vegfaa165) ck4 embryos without heat-shock (B) or with heat-shock (C). Strong induction of vegfaa165 can be detected upon heat-shock treatment. |
|
vegfaa expression was not altered in mib-/- embryos. (A and B) Expression of vegfaa was evaluated by whole mount in situ hybridization at 25 hpf in wild-type and mib-/- embryos.EXPRESSION / LABELING:
|
|
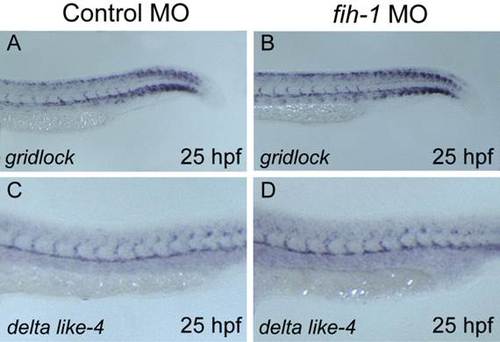
Endothelial notch target genes were not changed in fih-1 MO-injected embryos. (A–D) Expression of gridlock (grl) and delta lik-4 (dll4) were evaluated by whole mount in situ hybridization at 25 hpf in control and fih-1 MO-injected embryos. |