- Title
-
The Par-PrkC Polarity Complex Is Required for Cilia Growth in Zebrafish Photoreceptors
- Authors
- Krock, B.L., Perkins, B.D.
- Source
- Full text @ PLoS One
|
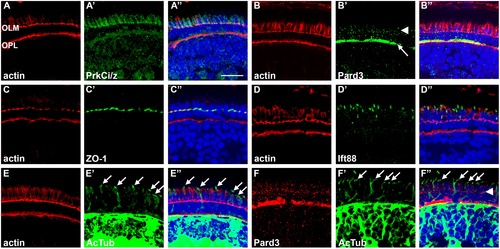
Spatial distribution of cell junction proteins and cilia in 5 dpf zebrafish. (A–E) Left-most panels show transverse cryosections stained with phalloidin (red) to label actin in the adherens junctions at the outer limiting membrane (OLM) and the outer plexiform layer (OPL). The middle panels show staining for determinants of apico-basal polarity (green), while the right-most panels show the merged images. (A1–A3) PrkCi/z immunoreactivity. (B1–B3) Pard3 immunoreactivity localizes to the OPL (arrow) and at cell junctions (arrowhead). (C1–C3) ZO-1 staining at the OLM. (D1–D3) Ift88 immunoreactivity stains cilia. (E1–E3) Acetylated tubulin immunoreactivity was seen in cilia and neural processes throughout the inner nuclear layer and plexiform layers. (F1–F3) Pard3 staining (red) was seen at the OLM (arrowhead), but did not colocalize with acetylated tubulin signal in the cilia (arrows). All sections were counterstained with DAPI (blue). Scale bar = 10 μm. EXPRESSION / LABELING:
|
|
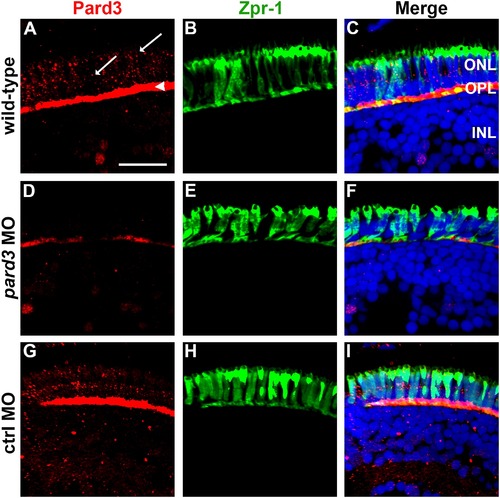
Pard3 expression is reduced at 5 dpf following injection of pard3 morpholinos. (A–C) Transverse cryosections of wild-type retinas, (D–F) pard3 morphant retinas and (G–I) control morphant retinas with Pard3 antiserum (left column) and the monoclonal antibody zpr-1 (middle column). The right panels show the merged images. Pard3 immunoreactivity was seen in the outer plexiform layer (OPL) and at cell junctions (A, arrowhead and arrows, respectively) in wild-type and control MO retinas but was significantly reduced in morphants retinas. All sections were also counterstained with DAPI (blue). ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer. Scale bar = 10 μm. |
|
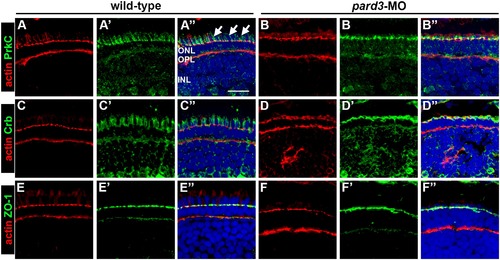
Effects of pard3 suppression on apical determinants. Immunohistochemistry was performed on transverse cryosections of wild-type and pard3 morphants. Phalloidin was used to label actin (red) in all panels. (A–B) Transverse cryosections of wild-type and pard3 morphants stained with phalloidin (red) and PrkCi immunoreactivity (green). Arrows mark longitudinal fibers in the ellipsoid. (C–D) Cryosections stained with phalloidin (red) and Crb immunoreactivity (green). (E–F) Cryosections stained with phalloidin (red) and ZO-1 immunoreactivity (green). Sections were also counterstained with DAPI (blue). ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer. Scale bar = 10 µm. |
|
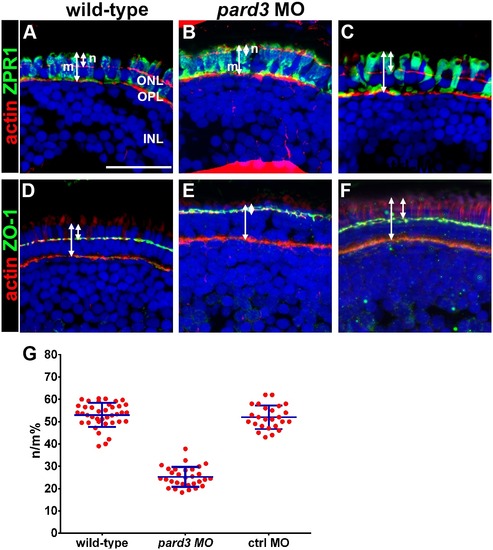
Apical domain size is reduced in pard3 morphants. (A–C) Transverse cryosections of 5 dpf retinas were stained with phalloidin to label actin (red) and zpr-1 to label double cones (green). The length of the photoreceptor (m) and the length of the apical region of the inner segment (n) are noted by arrows. (D–F) Transverse sections stained with phalloidin (red) and ZO-1 (green) to label the outer limiting membrane. Sections were also counterstained with DAPI (blue). (G) Quantification of apical domain as a percentage of total photoreceptor inner segment length. All values are plotted as a percentage of n/m, as defined in the text. Wild type = 53%; pard3 morphants = 25%; control morphants = 52%. Blue error bars indicate the mean ± standard deviation. ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer. Scale bar = 20 μm. |
|
Morpholino suppression of Pard3 causes variable cyclopia and loss of photoreceptor outer segments. (A–C) Transverse plastic sections of 4 dpf larvae heads from wild-type and pard3 morphants show variable degrees of cyclopia but normal lamination. (D–F) Higher magnification of the boxed areas in A–C revealed the photoreceptor outer segments in wild-type retinas (Panel D, arrows), which were missing in pard3 morphants. Morphants with fused eyes also exhibited patchy pigmentation in the RPE (Panel E, arrowheads). (G–H) Transmission electron microscopy of wild-type and pard3 morphant retinas showed truncation of photoreceptor outer segments. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer. Scale bar = 50 μm (A–C); 10 μm (D–F); 1 μm (G–H). PHENOTYPE:
|
|
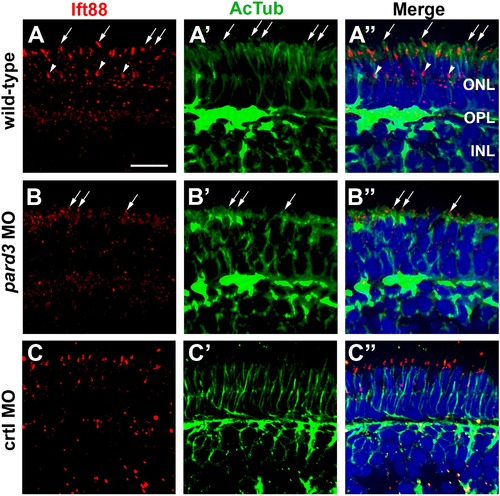
Connecting cilia are reduced in pard3 morphants. (A–C) Transverse cryosections were stained for Ift88 (left, red) and acetylated tubulin (middle, green) to label cilia in 5 dpf retinas. Merged images are shown in right panels. Ift88 localizes to the connecting cilia emanating from the apical surface of photoreceptors (arrows). Staining is also seen in connecting cilia of UV cones (arrowheads), which are tiered below the other cones. Sections were also counterstained with DAPI (blue). ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer. Scale bar = 10 μm. |
|
Rhodopsin is mislocalized in pard3 morphants. (A) Immunohistochemistry was performed on transverse cryosections through the retina of 5 dpf wild-type larvae. Left most panels show phalloidin staining to label actin (red). Rhodopsin immunoreactivity (green) is shown in middle panels. (D–F) In pard3 morphants, outer segments appear slightly shorter and opsin staining within the inner segment is observed (bracket). (G–I) Rhodopsin localizes normally in control morphants. Sections were also counterstained with DAPI (blue). ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer. Scale bar = 20 μm. |
|
Photo-Morpholinos reduce apical domain size. (A–D) Transverse cryosections of 4 dpf retinas were stained with phalloidin to label actin (red) and zpr-1 to label double cones (green). Sections were also counterstained with DAPI (blue). (E) Quantification of apical domain as a percentage of total photoreceptor inner segment length. All values are plotted as a percentage of n/m, as defined in the text. Wild type = 38%; pard3-pard3pMO morphants = 16%; prkci-prkcipMO morphants = 9.72%; prkc-prkcpMO was non-detectable. Blue error bars indicate the mean ± standard deviation. ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer. Scale bar = 20 μm. |
|
Photo-Morpholinos cause rhodopsin mislocalization and loss of rod photoreceptors. (A, E) Transverse retinal cryosections from 4 dpf wild-type; (B, F) pard3MO-pard3pMO; (C, G) prkciMO-prkcipMO; (D, H) prkci/zMO-prkci/zpMO injected larvae. Embryos were injected with MO/S-photo-MO hybrids and irradiated at 30 hpf with UV light. Immunohistochemistry was performed on transverse cryosections of 4 dpf larvae and stained with 1D1 to label rhodopsin (green) and phalloidin (red) and counterstained with DAPI to label nuclei. Bottom row shows 1D1 and DAPI staining only. (I) Quantification of total 1D1+ rod photoreceptors in 10 micron cryosections. (*** = p≤0.0001) ONL = outer nuclear layer; INL = inner nuclear layer. Scale bar = 100 μm (A–D) or 20 μm (E–H). |
|
Pharmacological inhibition of PrkC activity reduces apical domain size. (A–C) Transverse cryosections of 4 dpf retinas were stained with phalloidin to label actin (red) and zpr-1 to label double cones (green). (A) DMSO-control treated larvae; (B) Larvae treated with 3 μM PrkC inhibitor; (C) Larvae treated with 3 μM control inhibitor. Sections were also counterstained with DAPI (blue). (D) Quantification of apical domain as a percentage of total photoreceptor inner segment length. All values are plotted as a percentage of n/m, as defined in the text. Control = 45%; PrkC inhibitor = 28%; non-myristoylated control inhibitor = 44%. Blue error bars indicate the mean ± standard deviation. Scale bar = 10 μm. |
|
Pharmacological inhibition of PrkC activity reduces cilia length and causes opsin mislocalization. (A–C) Transverse cryosections of 4 dpf retinas were stained with phalloidin to label actin (red) and 1D1 to label rhodopsin (green). (D–F) Immunohistochemistry on cryosections to label Ift88 in cilia (red) and acetylated tubulin to label ciliary microtubules (green). Arrows identify cilia. Sections were also counterstained with DAPI (blue). (G) Quantification of cilia length. Cilia length in 4 dpf wild-type photoreceptors (43.2 microns) and the non-myristoylated control inhibitor (48.9 µm) were not statistically different from each other, but both were statistically different from larvae treated with the PrkC inhibitor (27.1 microns). Error bars show standard deviation. (***p≤0.0001) Scale bar = 10 μm. |
|
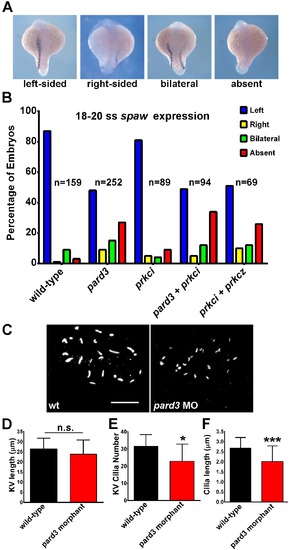
Left-right asymmetry requires pard3. (A) In situ hybridization for southpaw (spaw) expression in the lateral plate mesoderm in 18–20 somite stage embryos. pard3 morphants show left-sided, right-sided, bilateral, or an absence of spaw expression. (B) Graphical summary from three independent experiments showing the percentage of control and morphant embryos with left-sided (L), right-sided (R), bilateral (B), or absence (A) spaw expression. The number (n) of embryos analyzed is given next to each set of bars. (C) KV cilia in a wild-type and pard3 morphant. (D) Quantification of KV length across the longest axis. (E) Quantification of KV cilia number. (F) Quantification of KV cilia length. Bars show mean ± standard deviation (*p≤0.05; ***p≤0.0001) Scale bar = 20 μm. EXPRESSION / LABELING:
PHENOTYPE:
|