- Title
-
Descending Control of Swim Posture by a Midbrain Nucleus in Zebrafish
- Authors
- Thiele, T.R., Donovan, J.C., Baier, H.
- Source
- Full text @ Neuron
|
Enhancer Trap Line Gal4s1171t Drives Expression in the nMLF (A) Confocal projection of the midbrain in a 6-day-old Gal4s1171t/UAS:GFP fish. Optical sections (100 μm) were collapsed to yield a maximum intensity projection. Reticulospinal neurons were backfilled from the spinal cord with Texas Red dextran. Right panel is an expanded view of the nMLF region indicated by the red box in the left panel. Green cells are GFP-labeled by Gal4s1171t. Magenta cells are RS neurons in the rostral hindbrain and midbrain, labeled by Texas Red. White or pale magenta cells within the white circle are left nMLF neurons, which are double-labeled. (B) Confocal projection (80 μm) of the dorsal expression pattern in Gal4s1171t/UAS:GFP. The axon tract of the MLF (green arrowhead) and dendrites exiting the nMLF (orange arrowhead) are highlighted. (C) Two-photon image of a single plane highlighting four identified nMLF neurons, MeLr, and MeLc, plus the newly identified MeS1 and MeS2. (D) Confocal image projection of a cryostat section (25 μm) from Gal4s1171t/UAS:GFP stained with antibodies to GFP (green) and ChAT (red). Cell nuclei are labeled with a DNA dye (blue). See also Figure S1 and Movie S1. |
|
MeS Neuron Morphologies Revealed by Single-Cell Electroporations (A–C) Examples of confocal image projections of the midbrain in 6-day-old Gal4s1171t/UAS:GFP fish. MeLm (A), MeS type 1 (B), and MeS type 2 (C) are shown. Cells were electroporated with tetramethylrhodamine (TMR) dextran (magenta). GFP (green) is driven by Gal4s1171t. TMR is brighter than GFP in cellular processes. The dendrites therefore appear magenta. The somas of electroporated cells are double-labeled by GFP and TMR and appear as white. (D–F) Drawings of the nMLF cells in (A)–(C) showing their axonal projections. The corresponding photomicrographs are provided in Figure S2. Axon termination points are indicated by red arrowheads. Rostral collaterals in the hindbrain are indicated by blue arrowheads. (G) Summary table for all electroporations performed. See also Figure S2. |
|
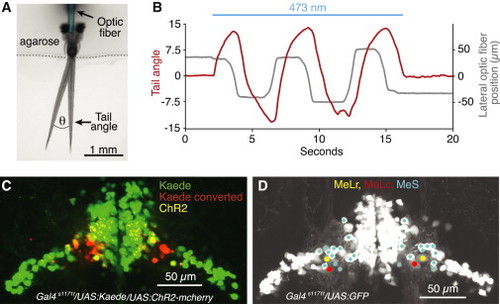
ChR2 Stimulation of nMLF Neurons and Their Localization Using Photoconversion (A) Dorsal view of the experimental setup used for ChR2 experiments. Two different tail positions are shown to illustrate the tail angle measurement used below and in subsequent figures. Optic fiber is pseudocolored for clarity. (B) Change in tail angle (red) in a Gal4s1171t/UAS:ChR2 fish, elicited by nMLF stimulation with a laterally moving optic fiber (gray; 10 µm fiber diameter, continuous beam 0.8 mW/mm2). Blue line depicts the epoch of ChR2 stimulation. (C) Confocal image projection of the midbrain in a Gal4s1171t/UAS:ChR2/UAS:Kaede fish, which underwent bilateral Kaede conversions at sites that produced left and right steering, respectively. (D) Confocal image projection, detailing the positions of MeLr, MeLc, and the MeS neurons in the lateral nMLF. See also Figures S4, S5, and Movie S3. |
|
Behavioral Effects of ChR2 Stimulation Depend on Light Dose and Fiber Location (A) Top: schematic of five stimulation sites within the midbrain expression pattern of Gal4s1171t. Bottom: traces depict tail angle as a function of time in a single fish at five stimulus locations with 10 Hz, 20 Hz, and 30 Hz light pulses (10 μm fiber diameter, 10 ms pulse duration, 1 mW/mm2). Blue line depicts the epoch of ChR2 stimulation. (B) Probability density histogram for maximum tail angles at left, middle and right stimulation sites (n ≥ 30 for left and right positions, n ≥ 20 middle). Color code is the same as in (A). (C) Mean values for maximum tail angle at left, middle and right stimulation locations (effect of frequency and location: p < 0.01). Color code is the same as in (A). (D) Probability of observing different tail kinematics at each stimulation frequency. Left and right trials were combined. (E) Tail beat frequencies for swim bouts at each stimulation frequency (effect of frequency: p < 0.05; n ≥ 9). (F) Two trials (blue and yellow) where long swimming bouts were evoked by bilateral stimulation with a large (105 μm) optic fiber (n = 2; 20 Hz pulse frequency). n values indicate number of fish. Error bars indicate SEM. See also Figure S6. |
|
Contributions of MeL Neurons and MeS Neurons to Steering (A) Two-photon image projection of the midbrain in a Gal4s1171t/UAS:GFP fish, in which the lateral region of the right nMLF was ablated 12 hr prior. Scale bar represents 50 μm. (B) Left: tail angle as a function of time after stimulating left (bottom traces) and right (top traces) ChR2 sites before and after lateral right nMLF ablation. Right: reduction in tail angle is observed on the ablated side (n = 14, paired t test, p < 105; t(13) = 6.91), but not on the control side (n = 14, paired t test, p = 0.12; t(13) = 1.62). Blue line depicts the epoch of ChR2 stimulation. (C) Left: tail angle as a function of time for left and right ChR2 stimulation sites before and after ablation of MeL neurons on the left side (bottom traces) and MeS on the right side (top traces). Right: reduction in tail angle is observed for MeL ablations (n = 7, paired t test, p < 0.02; t(6) = 3.49) and MeS ablations (n = 7, paired t test, p < 0.001; t(6) = 5.91). n values indicate number of fish. Error bars indicate SEM. Blue line depicts the epoch of ChR2 stimulation. |
|
Role of Lateral nMLF Neurons in the Optomotor Response (A) Schematic of the optomotor assay. Animal is head-embedded in agarose with the tail free. Two LCD screens on each side of the fish display caudal to rostral moving gratings for forward OMR stimulation. A third screen (not shown) is added in front of the animal to create rotating gratings for contraversive and ipsiversive OMR stimulation. (B) Change in tail angle in a Gal4s1171t/UAS:ChR2 fish elicited by continuous forward OMR stimulation (black) or by both OMR stimulation and ChR2 activation of the right nMLF (red). Blue line depicts the epoch of ChR2 stimulation. (C) Increase in ipsilateral tail angle during forward OMR swim bouts induced by ChR2 stimulation (n = 21; p < 10-6; t(20) = -5.26). (D) Change in tail angle in a Gal4s1171t/UAS:GFP fish evoked by three different OMR stimuli before (black) and after (red) ablation of lateral neurons in the left nMLF. All traces are from the same fish. (E) Projection of video frames displaying tail position of three fish (right ablated, control and left ablated) for an entire forward OMR trial. (F) Change in tail angle evoked by a forward OMR stimulus before (gray) and after (red) ablation of lateral neurons in the right nMLF. Black arrowhead denotes a left tail deflection that precedes swimming. Traces are from the same fish. (G) In ablated fish (red bars) swims are biased toward the intact side for forward OMR (n = 31; paired t test, p < 10-6; t(30) = 6.96). Turns evoked by contraversive (n = 28; p = 0.076; t(27) = 1.84) or ipsiversive (n = 28; p = 0.55; t(27) = 0.59) grating stimuli are unchanged. In control fish (gray bars), no biases were observed for forward (n = 10; p = 0.70; t(9) = 0.39), contraversive (n = 8; p = 0.52; t(7) = -0.67), or ipsiversive (n = 8; p = 0.86; t(7) = -0.17) stimuli. (H) Unilateral superficial small neuron ablations (blue bars) generated a bias in OMR forward swims toward the intact side (n = 13; paired t test, p < 0.02; t(12) = 2.82). Responses to contraversive (n = 11; p = 0.63; t(10) = 0.48) or ipsiversive (n = 11; p = 0.88; t(10) = -0.14) stimuli were unchanged. Unilateral MeL neuron ablations (red bars) did not affect forward swims (n = 8; p = 0.62; t(7) = 0.51), or turns to contraversive (n = 8; p = 0.97; t(7) = 0.03) or ipsiversive (n = 7; p = 0.63; t(6) = 0.51) stimuli. n values indicate number of fish. Error bars indicate SEM. See also Figure S7 and Movies S4 and S5. |
|
Specific Muscle Activation Generates Tail Deflections (A) Change in tail angle in Gal4s1171t/UAS:ChR2 fish elicited by ipsilateral hypaxial muscle stimulation with a stationary vertically oriented optic fiber (105 μm fiber diameter, continuous beam 2 mW/mm2). Blue line depicts the epoch of ChR2 stimulation. The blue trace represents the average change in tail angle when lateral muscle regions in the vicinity of myotomes 4–6 were stimulated (n = 6). The red trace represents the average change in tail angle when muscle regions just lateral to the midline were stimulated (n = 6). The green trace represents the average change in tail angle in Gal4s1171t/UAS:GFP control fish (n = 3). (B) Confocal image projection from the side and top of a Gal4s1171t/UAS:ChR2/UAS:Kaede fish that underwent Kaede conversion at a lateral muscle site, which produced a substantial tail deflection angle. This conversion labeled the posterior hypaxial muscle group (PHM) outlined in white. Numbers indicate myotome. (C) Confocal image projection from the side and top of a Gal4s1171t/UAS:ChR2/UAS:Kaede fish that underwent Kaede conversion at a medial muscle site, which produced a small, but detectable tail deflection. This conversion labeled the trunk hypaxial muscle group (THM) outlined in white. (D) Average calcium responses in ipsilateral PHM (blue), ipsilateral THM (red), contralateral PHM (blue dash), and contralateral THM (red dash) following unilateral nMLF optical stimulation in Gal4s1171t/UAS:ChR2/UAS:GCaMP6s fish (n = 5). Blue line depicts a 200 ms epoch of ChR2 stimulation. ChR2 was stimulated using the minimum laser power required to produce a tail deflection. n values indicate number of fish. PF, pectoral fin. See also Figure S8. |
|
Anatomy of the nMLF revealed by spinal cord backfills (related to Figure 1). A. Confocal image projection (80 μm) of several left nMLF neurons backfilled from the spinal cord with Texas Red dextran. In this backfill, the large identified neurons MeLc, MeLr, MeLm and MeM are prominently labeled. The identity of each cell is indicated. In addition, the newly identified smaller MeS1 and MeS2 cells are indicated. B. Two-photon image projection (80 μm) of a different nMLF backfill in which many MeS neurons are labeled as defined by their small soma diameter. C. Confocal image projection (220 μm) displaying both dorsal and ventral expression patterns in Gal4s1171t/UAS:GFP. |
|
MeLm and MeS axon morphologies revealed by single-cell electroporations (related to Figure 2). Single cell axon morphologies for a MeLm, MeS type 1 and MeS type 2 neuron. GFP expressing neurons in the region of the nMLF were targeted for electroporation in Gal4s1171t/UAS:GFP fish. Cells were electroporated with tetramethylrhodamine dextran 3,000 MW. These images were used for the axon tracing illustrations in Figure 2D-F. |
|
Kaede photoconversions (related to Figure 4). A. Confocal image projections (80 μm) for four Gal4s1171t/UAS:Kaede/UAS:ChR2 fish that underwent bilateral Kaede conversion at sites that evoked tail deflections. B. Confocal image projections (80 μm) for six Gal4s1171t/UAS:Kaede/UAS:ChR2 fish that underwent unilateral Kaede conversion at a site that evoked an ipsilateral tail deflection. |