- Title
-
Excitotoxic brain injury in adult zebrafish stimulates neurogenesis and long-distance neuronal integration
- Authors
- Skaggs, K., Goldman, D., Parent, J.M.
- Source
- Full text @ Glia
|
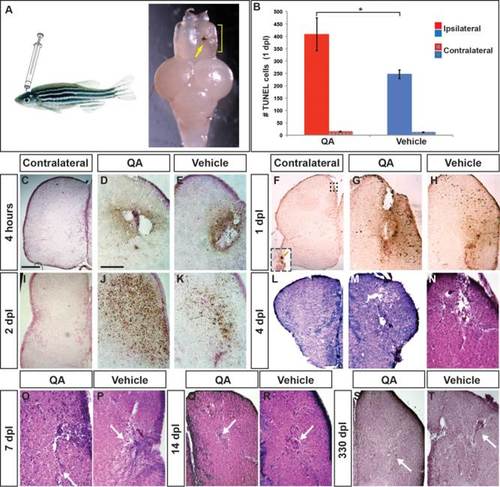
Telencephalic injury with QA results in early cell death followed by repair. (A) The adult zebrafish telencephalon was lesioned by injecting QA or vehicle orthogonally into the right hemisphere to a depth of approximately 2 mm. Two days postinjury, a blood clot identifies the injection track (arrow) and tissue disruption can be seen in the damaged hemisphere (yellow bracket). (B–K) Cell death was examined using a TUNEL assay. (B) The number of TUNEL-positive cells was quantified in the injured and uninjured hemispheres 1 day following QA or vehicle injection. Results from a one-way analysis of variance (ANOVA) indicated that both types of injury increased cell death, with more TUNEL-positive cells in the injured hemisphere following QA- than vehicle-induced injury (F(1,16) = 4.928, P < .05, n = 9). There was no difference in the number of TUNEL-positive cells on the contralateral side between QA and vehicle injuries (F(1,16) = 1.755, n.s.). (C, F, I) Very few apoptotic cells were seen on the uninjured side of the telencephalon following QA lesions (inset in F). (D,E) Dying cells appear near the injury site within 4 h after injury both in QA (D) and vehicle (E) conditions. (G,H) At 24 h postinjury the area of TUNEL reactivity has expanded in both QA- (G) and vehicle-injected (H) fish. TUNEL positive cells can be seen some distance from the injection site. (J,K) TUNEL positive cell numbers peak at 48 h postinjury, with a more extensive area of TUNEL reactivity in QA- (J) than vehicle-injured (K) brains. (L–T) H & E staining reveals extensive damage 4 days following both QA (M) and vehicle (N) lesions as shown by tissue loss at the site of injury and spongiform appearance in the surrounding areas. By 7 days post-lesion, tissue healing is apparent (white arrows in O, P), particularly following QA-induced lesions, and extensive repair is evident in lesioned tissue at 14 days post-lesion (white arrows in Q, R). More residual fibrous tissue was often seen following vehicle-induced lesions compared with QA-lesioned tissue. At 330 days post-lesion, morphological structure is largely intact in both QA- (S) and vehicle-lesioned (T) brains. Similar to early timepoints, fibrous remnants of vehicle-induced lesions are typically evident (arrow in T). Scale bar = 100 µm. Scale bar in C applies to F, I, L, M, S; scale bar in D applies to E, G, H, J, K, N, O, P, Q, R, T. |
|
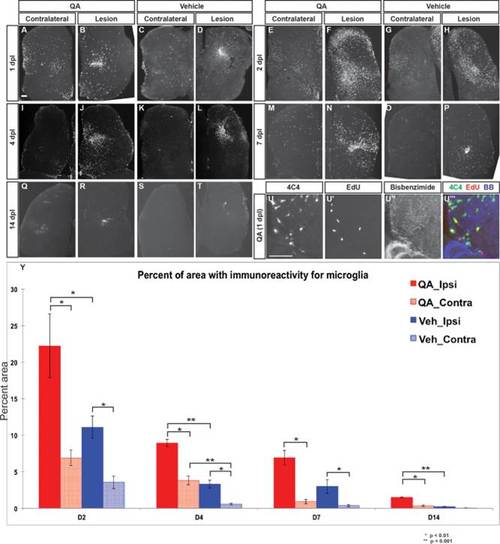
Microglial response to telencephalic injury is rapid and extensive. (A,D) Within 24 h post injury, 4C4-immunoreactive microglia appear in large numbers in the ipsilesional telencephalic hemisphere following both QA- (B) and vehicle-induced (D) lesions. Substantial numbers of microglia are also seen in the contralesional hemisphere (A, C) compared with the intact brain. (E–H) Microglial numbers peak at 48 h post injury in both the ipsilesional (F,H) and contralesional (E,G) hemispheres, with more widespread microglial presence in QA- (E-F, Y) relative to vehicle-induced (G,H,Y) injury. (I–T) The microglial response persists at lower levels on days 4 and 7 after injury, and returns toward baseline levels by 14 days. (U–U23) Microglia in the parenchyma proliferate as an early response to injury as shown by incorporation of EdU 24 h post-lesion. (Y) Quantification of the microglial reaction after QA or vehicle lesioning. The percent area of 4C4 immunoreactivity is more extensive following QA-induced than vehicle-induced injury in the ipsilesional hemisphere at all timepoints measured as well as in the contralesional hemispheres at 2, 4, and 14 dpl. ANOVA results indicated significant main effects for lesion type (F(1, 66) = 24.586, P < 0.0001), dpl (F(3, 66) = 37.330, P < 0.0001), and hemisphere (F(1, 66) = 46.988, P < 0.0001), with significant interaction effects for type of lesion x dpl (F(3, 66) = 3.577, P < 0.018), type of lesion x hemisphere (F(1, 66) = 6.051, P < 0.017), and dpl x hemisphere (F(3, 66) = 9.491, P < 0.0001). Significant post hoc analyses (Tukey HSD) were followed by pairwise comparisons with Bonferroni correction; significant results are indicated on the graph. Scale bar = 100 µm. |
|
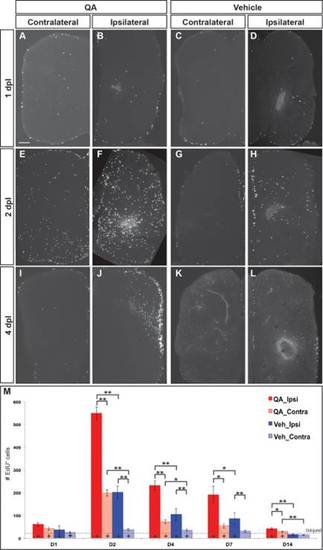
Cell proliferation increases dramatically following injury. (A–D, M) At 1 day post injury, proliferation began to increase in the VZs and parenchyma of the lesioned telencephalic hemispheres. (E–H,M) Extensive proliferation was evident in both ipsilesional and contralesional hemispheres by 2 dpl, especially in the germinative zones and throughout the parenchyma of the ipsilesional hemispheres after QA- more than vehicle-induced lesions. (I–L,M) The number of proliferative cells was elevated to a lesser extent at 4 (I–L) and 7 (M) dpl and remained only slightly increased at 14 days (M). (M) Proliferating cell numbers were quantified at various timepoints following QA- (red) or vehicle-induced (blue) injury in the ipsilesional (solid) and contralesional (hatched) telencephalic hemispheres. ANOVA results indicated significant main effects on proliferation for lesion type (F(2, 76) = 81.943, P < 0.0001) and dpl (F(8, 154) = 21.224, P < 0.0001), as well as a significant interaction effect for type of lesion x dpl (F(8, 154) = 14.482, P < 0.0001). Post hoc analyses (Tukey HSD) for proliferation across type of lesion indicated significant differences in proliferation in the injured hemisphere among QA-lesioned, vehicle-lesioned and uninjured brains, and in the uninjured hemisphere between QA-lesioned and vehicle-lesioned or uninjured brains. Results from pairwise comparisons within each day (Student′s t-test with additional Bonferroni corrections) and with uninjured telencephalic hemispheres are indicated on the graph and in Supporting Information Table 1. *, P < 0.01; **, P < 0.001; +, P < 0.01 compared with uninjured (denoted by the dashed horizontal line). Scale bar = 100 µm. |
|
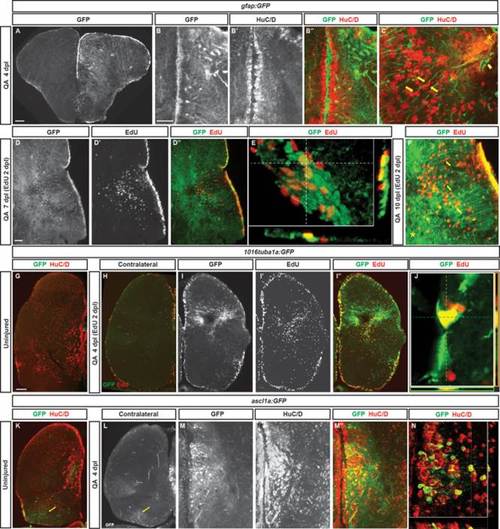
Radial glial-like progenitors respond to injury by proliferation and generation of new neurons. (A) Coronal section of the telencephalon of a QA-lesioned Tg(gfap:GFP) zebrafish shows activation of gfap manifest by GFP labeling in radial glial-like progenitors throughout the neurogenic regions in the injured hemisphere (right side of panel) in contrast to the low level of constitutive activity in the uninjured hemisphere at 4 dpl. (B–B′′) Expanded GFP expression (green) is seen in radial glial-like progenitors located in the VZ as well as hypertrophic GFP-expressing processes within the parenchyma in response to QA-induced lesion. HuC/D in red. (C) Double immunostaining for GFP (green) and HuC/D (red) in QA-lesioned brains at 4 dpl shows clusters of HuC/D+ neurons in close proximity to GFP+ processes that extend from the medial VZ to the injury (arrows indicate examples). These processes terminate in structures resembling endfeet near the lesion location (arrowhead). (D–D32) By 7 dpl, horizontal sections show that proliferative GFP+ (green) radial glial-like progenitors that incorporated EdU (red) 5 days earlier persist in the VZ, while EdU-labeled cells that appear to have migrated from the VZ to the injury site no longer express GFP (inset magnified in D′′′). (E) Orthogonal projection shows colocalization of GFP and EdU at 7 dpl. (F) At 10 dpl, costaining for EdU and GFP indicates that many of the newborn cells have migrated substantial distances toward the injury site from the lateral VZ, appearing in close proximity to or in contact with GFP+ processes (arrows indicate examples). (G–J) Tg(tuba1a:GFP) zebrafish show minimal GFP expression (green) in the uninjured telencephalon (G), increased expression mainly in the VZ of the contralesional hemisphere at 4 dpl (H), and massively increased GFP labeling in the lesioned hemisphere (I–I3) in both the VZ and parenchyma at the injury site. Many GFP+ cells were proliferating 2 days earlier (at 2 dpl) as indicated by EdU incorporation (I′′, J). (K–N) Tg(ascl1a:GFP) fish show a low level of VZ, periventricular and caudal (yellow arrows) GFP labeling (green) in the uninjured state (K) or contralateral to QA injection at 4 dpl (L). In the injured hemisphere, GFP expression increases markedly at 4 dpl in the VZ and periventricular region, and many of the cells coexpress HuC/D (M–N; red in K, M′′, and N). Scale bar = 100 µm. |
|
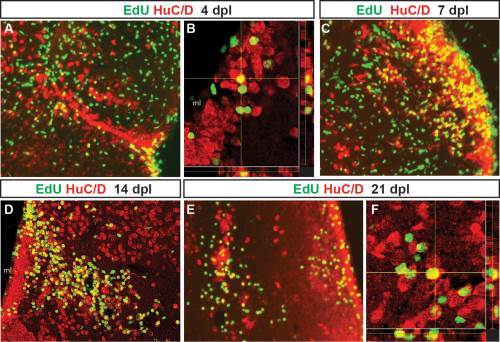
Adult-generated cells differentiate into neurons that progressively migrate to the site of injury. Proliferating cells that incorporated EdU at 2 days post-lesion were double labeled for EdU (green) and the neuronal marker HuC/D (red) after different chase periods. A yellow asterisk denotes the injury site in each panel. At 4 days after QA injection, EdU+ cells that express the neuronal marker HuC/D appeared to leave the VZ and extend toward the site of injury (A, orthogonal projection in B). By 7 dpl (C), EdU/HuC/D colabeled cells were found at a distance from the VZ. By 14 (D) and 21 dpl (E, orthogonal projection in F), two distinct populations of newborn neurons were present. Many double-labeled cells settled along the periventricular region where neuronal cell bodies are normally located. Other EdU/HuC/D double-labeled cells were located near the lesion site. Scale bars = 100 µm. |
|
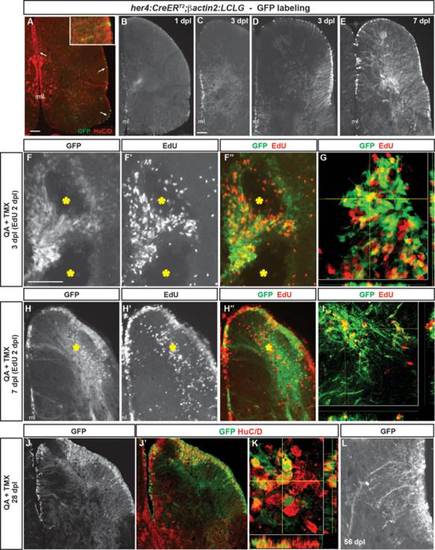
Radial glia-specific lineage mapping shows NPC activation, increased neurogenesis and migration to injury after QA-induced telencephalic lesioning. (A–A′′) A double transgenic zebrafish Tg(her4:CreERT2;β-actin2:LCLG) received tamoxifen intraperitoneally for 4 days and was examined 4 weeks later. Scattered GFP+ cells (green in A′′) appeared in the VZ (arrows) which generated a very small number of GFP+ neurons that extended a short distance from the ventricular surface and coexpressed the neuronal marker HuC/D (red; inset in A′′). (B) A modest amount of GFP expression was observed in the VZ at 1 dpl after intratelencephalic injection of QA and tamoxifen. (C,D) By 3 days after injury, the GFP+ progenitor population expanded in both the medial (C) and lateral (D) periventricular regions. (E) By 7 dpl, many GFP+ cell bodies were observed in the VZ, periventricular regions and parenchyma of the QA-lesioned hemisphere, indicating migration to injury, and an extensive network of GFP+ processes extended from the VZ to the lesion site. (F–F′′) Pulse-chase labeling with EdU (red in F′′) administered 2 dpl revealed that, as early as 1 day later, newborn cells derived from GFP+ radial glia extend toward the lesion site (yellow asterisks). (G) Orthogonal projection shows a cell colabeled with GFP and EdU that has migrated from the VZ. (H,I) Many GFP+ cell bodies that incorporated EdU at 2 dpl appeared in the parenchyma by 7 dpl while others remained in the ventricular/periventricular regions. Large numbers of GFP+ processes also extend from the ventricular region to the injury (yellow asterisk). (J,K) Many GFP+ cells (green) in both the parenchyma and periventricular regions remained at 28 dpl and coexpressed the neuronal marker HuC/D (red). (L) In contrast to their appearance as long, unelaborated processes at 3 dpl (see C,D), by 56 days after QA injection, GFP+ cells exhibit processes with highly branched, mature morphology. Scale bar = 100 µm. Scale bar in A applies to B. Scale bar in C applies to D, E, H, J. Scale bar in F applies to L. ml = midline. |
|
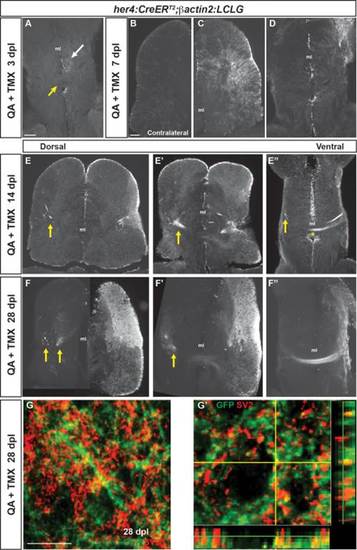
Neurons generated after QA-induced brain injury progressively extend processes to reestablish neuronal connections. (A) In the ventral telencephalon of Tg(her4:creERT2;β-actin2:LCLG) fish at 3 dpl, labeled progenitors began to extend GFP+ processes into the ipsilesional parenchyma (white arrow), but GFP expression was absent from the anterior commissure (yellow arrow) and contralateral hemisphere. (B–D) At 7 days after QA injection, minimal GFP expression was seen in the contralesional telencephalic hemisphere (B). In contrast, the ipsilesional hemisphere exhibited many GFP+ cells in the VZ and periventricular area that extended processes toward the injury site (C). At the level of the anterior commissure, GFP+ cells and processes were apparent but restricted to the ipsilesional hemisphere at 7 dpl (D). (E–E′′) Fasciculated bundles of GFP+ processes appeared by 14 dpl in the ipsilesional hemisphere and were also seen contralaterally (arrows) at dorsal levels (E, E2). More ventrally, GFP+ fibers appeared in the contralesional hemisphere (arrow in E3) and a GFP+ fiber bundle crossed at the level of the anterior commissure (E′′, asterisk). (F–F′′) The GFP+ bundles persisted at 28 dpl. At more dorsal levels, GFP+ fascicles were seen in the parenchyma of the contralesional hemisphere (F,F′), and at ventral levels an extensive fiber bundle crossed the anterior commissure (F′′). (G–G′) Colabeling for GFP (green) and the synaptic marker SV2 (red) in the contralesional telencephalon at 28 dpl revealed the appearance of synaptic contacts made by GFP+ axons onto putative targets. G′ shows an orthogonal projection of GFP+ synaptic contacts colabeled for SV2 (red). Scale bars = 100 µm. Scale bar in A applies to D, F′, F′&prime& in B to C, E, F. ml = midline. |
|
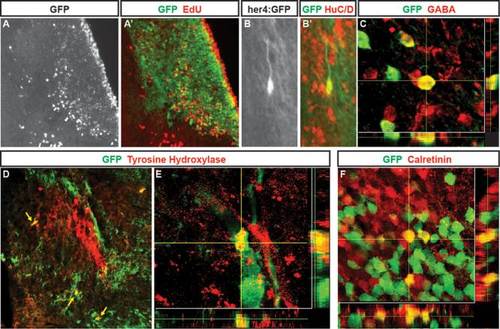
Neurons generated following QA-induced lesions of adult zebrafish telencephalon adopt multiple phenotypes. (A, A2) In Tg(her4:creERT2;β-actin2:LCLG) (her4:GFP) fish at 28 days post-lesioning, cells birthdated with EdU (A; red in A2) at 2 days after QA and tamoxifen injection have settled in the periventricular region and parenchyma of the damaged hemisphere where many coexpress GFP (green in A2). (B–B′) Higher magnification view of a GFP/HuC/D double-labeled cell with neuronal morphology in the parenchyma. (C) Neurons labeled for the inhibitory neuronal marker GABA include cells that coexpress GFP that comingle closely with other GABA-expressing cells. (D,E) Neurons double-labeled (yellow arrows) for GFP (green) and tyrosine hydroxylase (TH) comingle among the distinctive TH positive cluster of cells (red) in the ipsilesional hemisphere. (F) Within a calretinin-expressing group of neurons, some cells coexpressed calretinin (red) and GFP (green) whereas neighboring cells showed immunoreactivity for one of the two markers. Scale bar A, B, D = 100 µm. Scale bar C, E, F = 50 µm. |