- Title
-
Unc45b is essential for early myofibrillogenesis and costamere formation in zebrafish
- Authors
- Myhre, J.L., Hills, J.A., Jean, F., Pilgrim, D.B.
- Source
- Full text @ Dev. Biol.
|
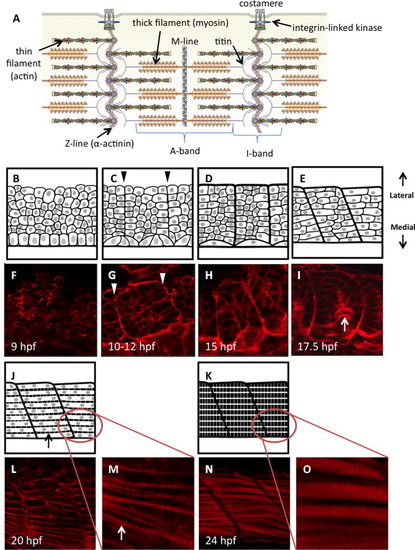
Diagrammatic representation of zebrafish somite maturation during myogenesis. (A) Schematic representation of the sarcomere in skeletal muscle, labeled to show the major protein components (modified from (Myhre and Pilgrim, 2012)). Panels B–E, J and K represent a dorsal view of paraxial mesoderm extending laterally from the edge of the developing neural tube (only the mesoderm is shown). Sample embryos at each stage are stained with phalloidin to show actin dynamics (F–I, L–O). Presomitic mesoderm in early embryos is made up of disorganized proliferative cells with thickened cortical actin structures (B, F). A columnar epithelium can be found immediately adjacent to the developing neural tube; these are the early adaxial cells. (C) Adaxial cells undergo apical constriction as the cells of the segmental plate align to create continuous actin barriers between the somites (arrowheads in C, G), and somites first become visible within the embryo. Fusion of adaxial cells results in the formation of the first contractile structures (D, H) containing slow-fiber myosin isoforms. Clockwise rotation of the somites begins at approximately the 15-somite stage, creating a curvature of the myosepta (E, I) that gives rise to the characteristic chevron shape. Concurrently, adaxial slow muscle fibers migrate laterally towards the somite periphery (arrow in I). At this stage, somite cells of the lateral paraxial mesoderm begin to elongate and fuse. Once somite cells are fully fused (J, L), striations appear in the cortical actin as the first myofibrils are constructed (arrows). As myocytes mature, they fill completely with striated myofibrils (K, N). Panels M and O show the formation of striated myofibrils at higher magnification. All artwork by Alina Pete. |
|
unc45b mRNA expression occurs long before muscle myosin expression, and correlates spatially and temporally with NMM. In situ hybridization of zebrafish embryos at the tailbud (A, E, I, M), 2 somite (B, F, J, N), 5 somite (C, G, K, O) and 10 somite (D, H, L, P) stages of development. In situ probes were generated against myh10 (A–D), unc45b (E–H). smyhc1 (I–L) and myhc4 (M–P). Non-muscle myosin (myh10) expression was detectable at the tailbud stage (A) in the bud itself, spreading throughout the neural tube by the 2-somite stage (B). Expression was also detectable in adaxial cells (arrows) and the lateral paraxial mesoderm (arrowheads), growing more pronounced from the 5-somite stage onwards (C, D). Expression of myh10 was not limited to the differentiating somites, extending throughout the presomitic mesoderm as well. Unc45b expression was detectable in presomitic adaxial mesoderm at the tailbud stage (E), extending to the tail from the 2-somite stage (arrow in F) onwards. Expression of unc45b in the lateral paraxial mesoderm was detected initially in differentiating somite cells only (arrowhead in F). However, from the 5-somite stage onwards (G, H), unc45b was detectable throughout all paraxial mesoderm (arrowheads) in both differentiating somites and caudal presomitic mesoderm (G2, H2, white arrowheads). By contrast, the earliest slow-muscle myosin (smyhc1) was not significantly expressed until the 5-somite stage (K), and was restricted to adaxial cells as expected (arrowheads in J–L). Expression extended into the first few presomites (white arrowheads in K and L) but not to the caudal-most presomitic tissue. Likewise, fast-muscle myosin (myhc4) was not detectable at all before the 5-somite stage, and was also restricted initially to adaxial cells (arrowheads in O and P), extending into the first few presomites (white arrowhead in O), but not to the caudal-most tissue (white arrowhead in P2). Prime labels indicate lateral views of the same embryo. Arrowhead positions are the same between dorsal and lateral views. EXPRESSION / LABELING:
|
|
unc45b mRNA expression correlates to non-muscle myosin but not muscle myosin in paraxial mesoderm. In situ hybridization, high magnification view of developing somites in zebrafish embryos at the 2-somite, (A, D, G, J), 5-somite (B, E, H, K) and 10-somite (C, F, I, L) stages. Non-muscle myosin (myh10, A–C) and unc45b (D–F) were expressed consistently in both adaxial cells (arrows) and lateral paraxial mesoderm (arrowheads) at all stages. In contrast, slow-muscle myosin (smyhc1, G–I) was expressed only in adaxial cells (arrows) at these stages, beginning lateral migration into the somites only at the latest stages examined (white arrowhead in I). Fast-muscle specific myosin (myhc4, J–L) was expressed after the 5-somite stage, and only in adaxial cells (arrows). EXPRESSION / LABELING:
|
|
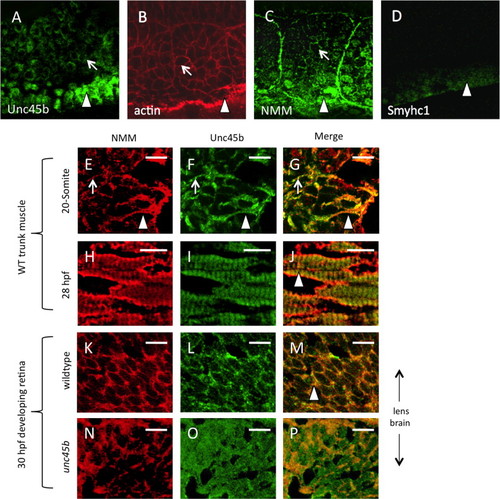
Unc45b protein is co-localized with non-muscle myosin protein in early paraxial mesoderm. Immunofluorescent staining of 5-somite (A–D) zebrafish embryos demonstrates protein accumulation of Unc45b (A) and non-muscle myosin (C) in both adaxial cells (arrowheads) and lateral paraxial mesoderm, demonstrating enrichment at the myoblast cortex (arrows) and somite boundaries, where cytoskeletal actin is also enriched (B). By contrast, muscle myosin protein accumulation (D) was limited to adaxial cells that form the superficial layer of slow muscle fibers. In cryosections of developing zebrafish muscle tissue (E–J), partial co-localization of Unc45b (green) and NMM (red) protein can be seen as yellow fluorescence within myoblast cortices (panels G and J). At the 20-somite stage (E–G), localization to myoblast cortices (arrows) and nascent myofibrils (arrowheads) are both evident. This partial co-localization persists at 28 hpf (H–J). In developing retinal tissue near the lens, Unc45b (K) and NMM (L) can also be seen to co-localize at 48 hpf (yellow fluorescence in panel M); however, this co-localization was lost in unc45b mutant embryos lacking the C-terminal myosin-binding UCS domain (N–P). Merged NMM and Unc45b staining is shown in panels G, J, M and P. Scale bars=10 µm. EXPRESSION / LABELING:
|
|
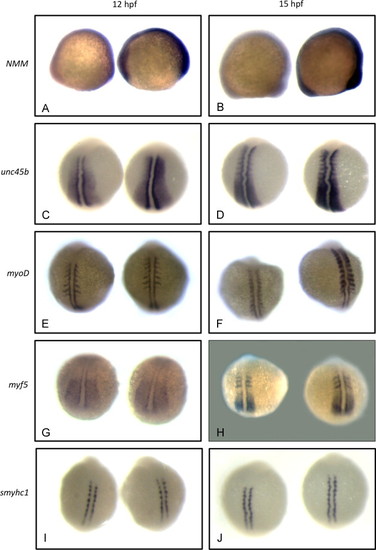
Non-muscle myosin and unc45b mRNAs are up-regulated in early unc45b mutant embryos. In situ hybridization of embryos at the 5-somite (A, C, E, G, I) and 10-somite (B, D, F, H, J) stages. Approximately 50 embryos were examined for each stage and marker. WT and unc45b mutant embryos were identified by dCAPS genotyping. Color development in unc45b mutant embryos (right-hand embryo in all panels) was much more rapid than in WT embryos (left-hand embryo in all panels) for non-muscle myosin myh10 (A, B), indicating up-regulation of mRNA expression. This increase is concurrent with the up-regulation of unc45b mRNA expression (C, D). RNA expression of myogenic regulators myoD (E, F) and myf5 (G, H) were not increased detectably in mutants at the 5-somite stage (compare left and right embryos in panels E and G). At the 10-somite stage, myf5 remained unaffected (H), but myoD mRNA expression was somewhat increased in unc45b mutants (F), concurrent with the onset of significant muscle myosin expression. smyhc1 mRNA expression was not affected at either stage (J, I). EXPRESSION / LABELING:
|
|
Non-muscle myosin and Unc45b localize with costamere components at myoblast cell peripheries and at myosepta. Immunofluorescent staining of early zebrafish embryos (10–12 hpf) demonstrates the localization of early sarcomere components (including non-muscle myosin) to the cortices of myoblasts during somite organization (depicted in top left panel). Myocyte costamere components such as α-actinin and integrin-linked kinase (ILK) localize to the myoblast cell cortex prior to costamere formation (arrowheads). Punctae in these panels indicate nucleation of costamere components at cell attachment sites (arrows). NMM and Unc45b are similarly localized to myoblast cortices. Phalloidin staining against actin marks the myoblast cortex. Subsequently, at 12–15 hpf (lower panels), costamere components organize at the myosepta (arrowheads) as depicted schematically in bottom left panel, including α-actinin and ILK. Non-muscle myosin and Unc45b were also localized to the developing myosepta (bottom right panels). EXPRESSION / LABELING:
|
|
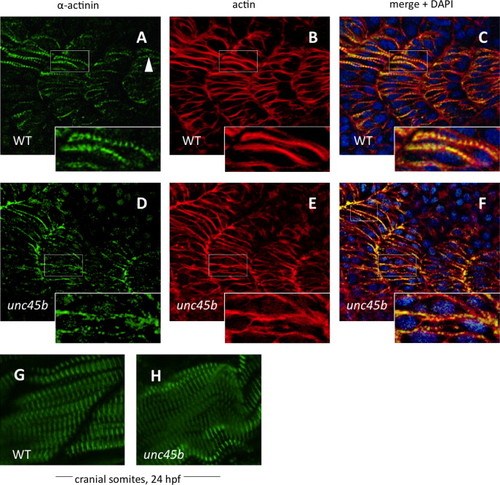
Nucleation of α-actinin is delayed in zebrafish unc45b mutants. Immunofluorescent staining of WT (A–C) and unc45b mutant embryos (D–F) at 20 h post-fertilization, displaying the 19th to 22nd somites. The caudal-most somites of these embryos were still undergoing myogenesis, as shown by the incomplete elongation of myofibers and localization of α-actinin and actin staining in WT embryos (A and B, inserts). Nucleation of α-actinin is the first indication of periodic myofibril patterning (arrowhead). In mutant embryos, organization of α-actinin at costamere attachment sites was not yet complete, and nucleation had just begun (D, insert). Actin counter-staining with phalloidin (B, E) demonstrates the ongoing organization of actin in early myofibers, which can be compared with the pattern of α-actinin localization in merged images (C, F). Blue fluorescence indicates DAPI nuclear stain. The cranial-most somites at 24 hpf display relatively normal patterns of α-actinin staining in mutants (H) compared to WT embryos (G). EXPRESSION / LABELING:
PHENOTYPE:
|
|
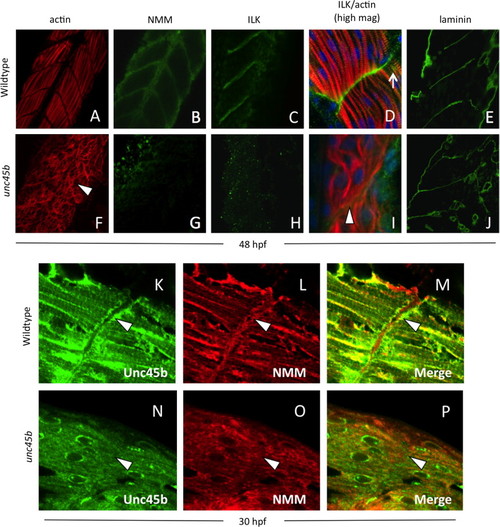
Localization of Unc45b, NMM and costamere components to the myoseptum is lost in unc45b mutant embryos. Immunofluorescent staining of WT (A–E) and unc45b mutant embryos (F–J) at 24+ hours post-fertilization. The positions of myofiber attachment points at the myosepta were made visible by actin staining (A, F). Like early embryos, mature muscle tissue was characterized by the localization of non-muscle myosin (B) and integrin-linked kinase (C, D) to the myosepta in WT embryos. This localization was lost in unc45b mutants (G, H, I). At higher magnification (D, I), ILK co-localizes with actin striations at lateral costamere attachment points (arrow in D); this pattern of staining was also lost in unc45b mutant embryos (I). Green fluorescence to the right of the actin staining represents background staining outside of the muscle tissue, and blue fluorescence indicates DAPI counter-staining. However, the tissue morphology was not changed in mutants, and the disorganized myofibers were still separated by myosepta (arrowhead in I) containing extracellular matrix components such laminin (compare panels E and J). Furthermore, enrichment of Unc45b (K) and NMM (L) at the myosepta in older embryos (arrowheads) was lost in unc45b mutants (N, O), and co-localization of these proteins was greatly reduced (compare M and P). |
|
|
Reprinted from Developmental Biology, 390, Myhre, J.L., Hills, J.A., Jean, F., Pilgrim, D.B., Unc45b is essential for early myofibrillogenesis and costamere formation in zebrafish, 26-40, Copyright (2014) with permission from Elsevier. Full text @ Dev. Biol.