- Title
-
Loss of FTO antagonises Wnt signaling and leads to developmental defects associated with ciliopathies
- Authors
- Osborn, D.P., Roccasecca, R.M., McMurray, F., Hernandez-Hernandez, V., Mukherjee, S., Barroso, I., Stemple, D., Cox, R., Beales, P.L., and Christou-Savina, S.
- Source
- Full text @ PLoS One
|
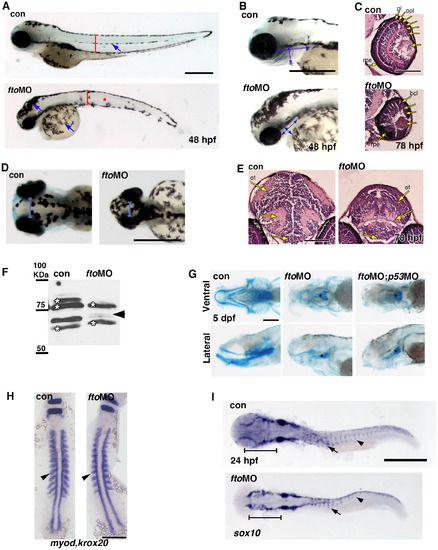
Loss of fto results in a craniofacial zebrafish phenotype. (A,B) fto knockdown zebrafish display small eyes, shortened dorsal ventral axis (red brackets), reduced pharyngeal width and length (double headed blue arrows), mislocalised melanocytes (blue arrows and red asterisk) and a curved truncated body axis. Scale bar: 500 µm, n = con 50/50, ftoMO 48/50. (C) Hematoxylin and Eosin staining on paraffin sections highlight loss of lamination and reduced size of the eye in fto morphants at 78hpf (n = con 10/10, ftoMO 10/10. Scale bar: 100 μm. pl; photoreceptor layer, opl; outer plexiform layer, bcl; bipolar cell layer, acl; amacrine cell layer, ipl; inner plaxiform layer, gcl; ganglion cell layer, rpe; retinal pigmented epithelium. (D) Dorsal whole mount view of control uninjected and fto morphant embryos at 48 hpf, morphants have reduced optic spacing (blue brackets) indicative of microcephaly. (E) Hematoxlyin and Eosin staining on transverse paraffin sections through the brain at eye level showing microcephaly in morphant embryos at 78 hpf. Scale bar: 100 μm. ot; optic tectum, t; tegmentum, h; hypothalamus. (F) Fto knockdown was confirmed in 48 hpf morphant embryos by western analysis using an anti-human FTO antibody, note the missing band at approximately 65KDa (arrow head). Asterisks indicate non-specific bands. (G) Embryos treated with the fto MO fail to develop the majority of head cartilage at 5 dpf compared to untreated controls, a reduced basal plate remains intact between treatments (asterisks). p53 MO was used to counteract off-target morpholino effects, p53 MO failed to rescue the ftoMO phenotype. Ventral and lateral views displayed in the top and bottom columns, respectively. Scale bar: 200 μm; n = con 30/30, ftoMO 36/41, ftoMO;p53MO 28/32. (H) In situ hybridisation for myod and krox20 in control and fto morphants at 14 hpf. Arrowheads indicate a reduction in somite size in morphants compared to controls. Scale bar 200 µm; n = con 52/55, ftoMO 45/48. (I) Aberrant migration of NCCs, visualised using sox10 probe, was observed in the head (brackets) and trunk (arrows) of fto morphants. Scale bar: 500 μm; n = con 33/33, ftoMO 27/34. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Canonical Wnt signaling is downregulated in fto morphants zebrafish. (A) Dual luciferase assay using the β-catenin responsive TopFlash construct shows loss of reporter assay activity in ftoMO embryos analysed at both 24 hpf (con: 1.000 SEM ±0.073, ftoMO: 0.491 SEM ±0.105) and 48 hpf (con:1.000 SEM ±0.103, ftoMO 0.580 SEM ±0.066) stages. (B) Lef1 transcripts, a canonical Wnt target gene, were analysed by in situ hybridisation (ISH) at 48 hpf. Fto morphants showed marked loss of lef1 expression in the optic-tectum (arrows). Scale bar: 500 μm. n = con 56/66, ftoMO 40/53. (C) Loss of β-Catenin was confirmed in fto morphants by western blotting at 48 hpf. β-Catenin protein levels were quantified relative to the loading control (Actin). (D) ISH analysis of ctnnb1 (zebrafish β-catenin 1) at 48 hpf showed upregulation of transcripts specifically in areas of the lateral hindbrain (arrowheads). Scale bar: 500 μm. n = con 70/70, ftoMO 65/68 (E) Fto morphant Tg(7xTCF-Xla.Siam:GFP)ia4 display loss of GFP accumulation in both the Telen- and Diencephalic regions of the brain when compared to uninjected controls at 48 hpf, embryos viewed from a dorsal perspective. Scale bar: 100 µm; n = con 20/20, ftoMO 18/20. |
|
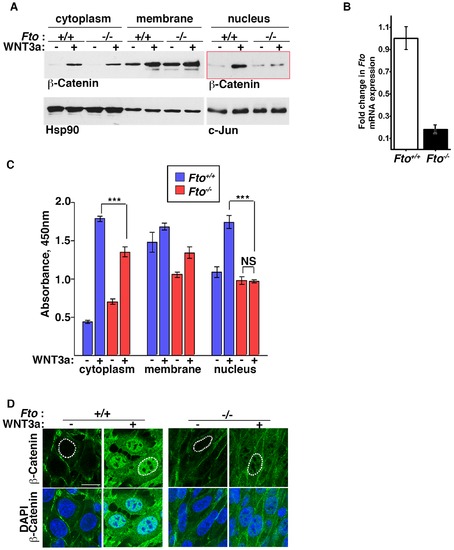
β-Catenin dependant canonical Wnt signaling is compromised in Fto deficient cells. (A) Cytoplasmic, membrane and nuclear fractions of control (Fto+/+) and Fto knockout (Fto/) MEFs treated with control () or Wnt3a -conditioned medium (+) for 4 hours were analysed by Western blot using β-Catenin antibody. Hsp90 and c-Jun were used as loading controls for cytoplasmic and nuclear fractions, respectively. (B) Fto mRNA expression in control and Fto knockout MEFs as determined by RT Real Time PCR. The data shown represent the mean ±SEM, (n = 3) (C) Quantification of cytoplasmic, membrane and nuclear β-catenin by ELISA in control and Fto knockout MEFs treated with control or Wnt3a -conditioned medium for 4 hours. The data shown represent the mean ±SEM, (n = 5). One-way ANOVA with Tukey’s multiple comparison test was performed, ***P<0.05, NS: not significant. (D) Immunofluorescence of control and Fto knockout MEFs treated with control and Wnt3a conditioned medium for 4 hours. Scale bar indicates 20 μm. This image is representative of three separate experiments. |
|
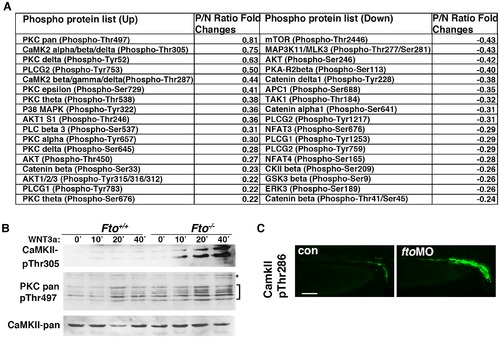
Ca2+/Wnt signaling is activated in Fto deficient cells and zebrafish. (A) Wnt signaling phospho antibody microarray.Control (Fto+/+) and Fto knockout (Fto/) MEFs were treated with Wnt3a and changes in phosphorylation of Wnt signaling proteins analysed by an antibody microarray. P/N: Phospho-Antibody/Non-Phospho-Antibody ratio. For detailed calculations see Methods. (B) Total CamKII, phosphorylated (Thr305) CaMKII, and pan phosphorylated PKC (Thr497) were analysed in control (Fto+/+) and Fto knockout (Fto/) MEFs treated with Wnt3a conditioned medium (+) for 0, 10, 20 and 40 minutes. Brackets indicate PKC isoforms, asterisks show a non-specific band. (C) Phosphorylated CaMKII (Thr287) is upregulated in the pronephric ducts (PND) of fto morphant embryos compared to uninjected controls, as shown by immunofluorescence at 48 hpf. Scale bar: 50 μm. EXPRESSION / LABELING:
|
|
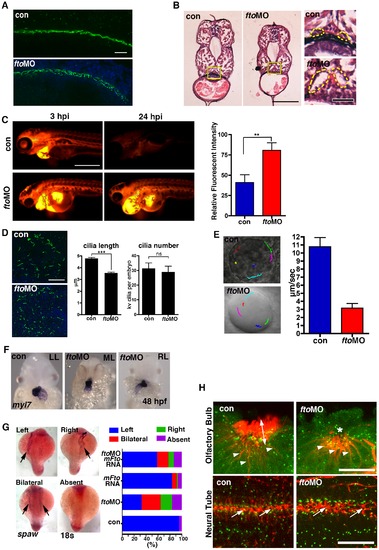
Loss of Fto in zebrafish leads to developmental defects associated with cilia dysfunction. (A) Cilia in the pronephric ducts of fto morphants are disorganised compared to wild type controls at 24 hpf. Cilia are marked by anti-acetylated tubulin (Green) and nuclei by DAPI (Blue); n = con 40/40, ftoMO 38/40 (B) Haemotoxylin and Eosin staining of wax sections through the pronephric ducts (at the level of the yolk extension) of control and fto morphants at 72hpf. Fto morphants develop dilated pronephric tubules, consistent with cilia disorganisation, highlighted by dotted lines in high magnification images to the right of the panel. Yellow squares demark magnified areas. Scale bars: 100 μm and 20 μm, low and high magnification images respectively. n = con 10/10, ftoMO 10/10. (C) Morphants have defective renal filtration as assayed through rhodamine dextran clearance assay. Images to the left of the panel depict embryos 3 hours post injection of the fluorescent tracer into the pericardium, the right side panels show the same fish 24 hpi, fto morphants retain significantly more fluorescence compared to controls, quantified and displayed as relative fluorescent intensity in graphical format (con: 40.98± SEM 9.539, n = 10. ftoMO: 80.54± SEM 9.516, n = 10. ** P<0.05). (D) Immunofluorecence of cilia, marked by anti-acetylated tubulin, and nuclei, DAPI, in the KV of 10 somite control and fto morphants. Morphants display shorter cilia than uninjected controls (con: 4.789±0.08773 N = 345, ftoMO: 3.557±0.06656 N = 464, *** P<0.0001) but have normal numbers of KV cilia per embryo (con: 31.36±3.757 N = 11, ftoMO: 29.00±3.896 N = 16, P = 0.6790, ns: not significant). (E) Fluorescent beads implanted into the KV at 12 hpf show that despite morphants displaying normal anticlockwise fluid flow, velocity of flow was significantly slower (con: 10.8 µm/sec ±1.1, fto MO: 3.2 μm/sec ±0.6, n = 5, movies in supplementary material). (F) In situ hybridisation for myl7 in control and fto morphants at 48 hpf show knockdown of Fto results in aberrant left-right patterning as observed by left looping (LL: 34/46), midline looping (ML: 5/46), and right looping (RL: 7/46) of the heart. (G) In situ hybridisation of spaw at 18 somite stage showing laterality associated defects in fto morphants that can be rescued when co-injected with mouse Fto RNA, see graph. (H) Acetylated- (red) and γ-Tubulin, marking the cilia and basal bodies respectively. FtoMO embryos show loss of cilia in the olfactory pit (upper panels). Arrow heads: olfactory neurons, double headed arrow: olfactory cilia, asterisk: reduced/absent olfactory cilia. Highly disorganised cilia in the neural tube (lower panels) of fto morphants at 24 hpf (arrows). Scale bar: 20 μm. N = con 10/10, ftoMO 23/31. PHENOTYPE:
|
|
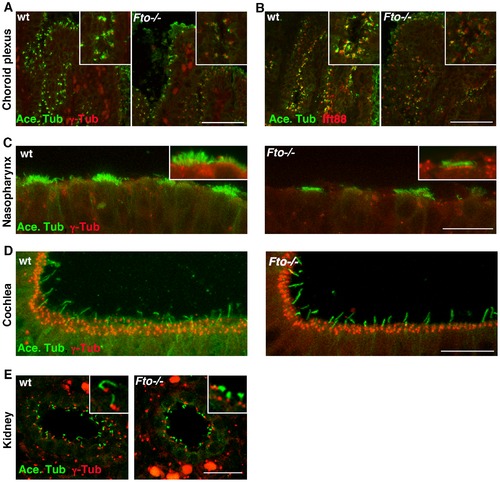
E15.5 Fto knockout mice embryos display tissue specific cilia defects. Paraffin sections from wild type and mutant animals showing immunolocalization of acetylated- α-tubulin (green) and γ-tubulin or IFT88 (red) in the choroid plexus (A,B); nasopharynx (C); cochlea (D); kidney (E). Loss of Fto results in shortened cilia in the choroid plexus, nasopharynx and kidney whilst cilia in the cochlea appear unperturbed. Scale bars: in A, B 50 μm; in C,D,E 20 μm. |
|
A second non-overlapping morpholino, against the exon3-intron3 splice site (fto spl.MO), confirms specificity of the fto phenotype. (A) fto spl. morphants show a similar general morphology to fto ATG morphants, displaying small eyes, reduced pharyngeal length, and curved truncated body axis at 48 hpf and 5 dpf. Scale bar: 500 μm. (B) Craniofacial defects were also observed in the fto spl. morphants, as in fto ATG morphants, assayed using alcian blue to detect cartilage. Scale bar: 200 µm. (C) RT-PCR of a product surrounding the E3-I3 splice site confirmed fto knockdown and specificity of the fto Spl.MO at 48 hpf, presumably due to the two in-frame intronic stop codons, 72 nt and 84 nt into intron 3, causing RNA mediated decay. GAPDH was used as a loading control. |