- Title
-
Abnormal differentiation of dopaminergic neurons in zebrafish trpm7 mutant larvae impairs development of the motor pattern
- Authors
- Decker, A.R., McNeill, M.S., Lambert, A.M., Overton, J.D., Chen, Y.C., Lorca, R.A., Johnson, N.A., Brockerhoff, S.E., Mohapatra, D.P., Macarthur, H., Panula, P., Masino, M.A., Runnels, L.W., and Cornell, R.A.
- Source
- Full text @ Dev. Biol.
|
The TRPM7-like current is dramatically reduced in trpm7b508 mutants. Representative whole-cell current recordings of melanophores isolated at 32 hpf, in (A) control or (B) trpm7 mutant embryos, in response to voltage ramps from -100 mV to +100 mV for 500 ms. (A) Control melanocytes exhibited outwardly rectifying currents that are potentiated by the extracellular application of 10 mM NH4Cl, and inhibited by the application of 10 mM MgCl2, properties that are characteristic of TRPM7-mediated currents. (B) The mutant melanophores did not exhibit any TRPM7-like outwardly rectifying currents. The slight outward rectification of currents in mutant melanophores indicates that the cells were alive. Control, n=7 cells; mutant, n=3 cells. PHENOTYPE:
|
|
The hypomotility of trpm7 mutants is levodopa responsive. Bar chart showing results of automated analysis of motility was carried out during daytime, over one cycle of 30 min exposure to light (white horizontal bar) followed by 30 min of darkness (black horizontal bar). Vertical bars indicate average distance moved during 5 min intervals. The average movement of mutants over the hour was significantly lower than that of controls (Welch t-test, p<0.001). For each experiment, half of a single clutch of larvae from trpm7 heterozygous adults was treated with 30 µM L-dopa for 3 h before recording. The motility of trpm7 mutants was significantly increased by L-dopa treatment during the first 10 min after the light-to-dark shift (Student′s t-test, p=0.004 and Welch t-test, p=0.017 for the first and second 5 min intervals after the light-to-dark transition, respectively). By contrast, control larvae were unaffected by L-dopa (Student′s t-test, p=0.097 and p=0.070 for the first and second 5 min intervals after the light-to-dark transition, respectively). Each group tested consisted of 22-24 animals. Error bars represent SEM in one single hr. Asterisks, values significantly different. PHENOTYPE:
|
|
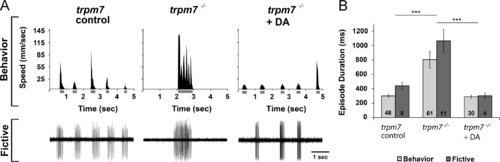
Dopamine rescues behavioral and fictive locomotor patterns of the trpm7 mutant. (A) (Top row) Representative recordings of speed and (bottom row) extracellular peripheral nerve recordings in fictive swimming preparations for discrete swimming episodes of 5-6 dpf control siblings (left), trpm7 mutants (middle), and trpm7 mutants+DA (20 µM; right). Grey bars below x-axes of speed plots represent episode durations whose beginnings and ends are defined by the filter settings. (B) Summary plot of the mean durations of behavioral and fictive swimming episodes for each group. Asterisks indicate significant difference; ***p<0.001. Numbers at the base of bars in graph denote number of larvae observed for each experimental group. Error bars represent SEM. PHENOTYPE:
|
|
trpm7 mutants are sensitized to MPP+. (A–H) Dorsal views of 5-dpf larvae of the indicated genotype, treated with 500 μm MPP+ or vehicle control as indicated. Individual panels show distinct groups of Th1 IR cells in the following brain regions: (A–D) pretectal diencephalon (group 7); (E–H) periventricular hypothalamus (group 13); and I and J) bar chart showing average numbers of Th1 IR-positive cells in each group (n=11 animals scored). In the untreated samples, the number of Th1 IR cells was significantly lower in mutants than controls in group 7 (control avg. +/- SEM: 44 +/- 2, mutant: 36 +/- 2; Two way ANOVA with Bonferroni post-hoc test, p<0.001).. In group 13, (control: 56 +/- 1, mutant: 51 +/- 2) there was not a significant difference in the number of Th1 IR cells between untreated controls and untreated mutants. In both groups, MPP+ treatment effected a larger reduction in the number of Th1 IR cells in mutants than in controls (group 7, control+MPP+: 39 +/- 2, mutant+MPP+: 17 +/- 2; group 13, control+MPP+: 53 +/- 2, mutant+MPP+: 37 +/- 3). In group 7 the effect of MPP+ in mutants was almost three times larger than in controls, and in group 13 it was over four times larger; in both groups, mutants were significantly more sensitive to MPP+ treatment than controls (p<0.001). (K and L) Dorsal views of dissected brains from (K) control or (L) trpm7 mutant larvae, processed to reveal th2 mRNA. The pre-optic area (group 3b) is outlined in white. (th2-positive cells: control: 14 +/- 1, n=9; mutant: 7 +/- 5, n=12; p<0.001). Scale bar in A=50 μm, applies to A-H. Scale bar in K, =100 μm, applies to K,L. (M) Schematic illustrating the numbering scheme of Th1 and th2 positive (outlined in black) neuron clusters where darkness indicates more ventral groups (1 olfactory bulb; 2 subpallium; 3–4 preoptic area; 5,6,11 diencephalon; 7 pretectum; 8 anterior paraventricular organ; 9 interior paraventricular organ; 10 posterior paraventricular organ; 12 posterior tuberal nucleus; 13 hypothalamus; 14 locus coeruleus; 15-16 medulla oblongata; 17 area postrema; see Table S1 for additional details) EXPRESSION / LABELING:
|
|
Numbers of serotonergic neurons and neurons expressing dat are normal in trpm7 mutants (A,B; A2,B2) Dorsal views of the (A and B) pre-tectum (Th1 group 7) and (A2 and B2) hypothalamus (Th1 group 13) in 5 dpf control and trpm7 mutant larvae, as indicated, processed to reveal anti-5HT (red) and anti-Th1 (green) immunoreactivity (IR). Th1 IR cells were reduced in mutants (quantified in Fig. 4), 5HT IR cells were normal in number (below). Scale bar in A=30 μm and applies to A–D; scale bar in A2=50 μm and applies to A2–B2 and E, F. (C and D) Dorsal views of 5 dpf Tg(dat:gfp) larvae focused on the pretectum (group 7). GFP-expressing neurons were equally abundant in trpm7 mutants and controls (control: 65 +/- 7; mutant: 60 +/- 10). (E and F) Ventral view of hypothalamus (group 13) in embryos of the indicated genotype processed to reveal dat expression by in situ hybridization. (G) Bar chart indicating numbers of 5HT IR and GFP-expressing neurons within the pretectum (group 7) of trpm7 mutants (or trpm7 mutant; Tg(dat:gfp) transgenic) and controls. Number of neurons was not significantly different between the genotypes. (Anti-Hu IR-positive cells in DRG caudal to hindyolk at 5 dpf, control: 32± 0, mutant: 31±1). (H) qRT-PCR analysis of mRNA levels of th1, th2, dat, and bdnf. Asterisks indicate significant difference; Np<0.001. Error bars represent SEM. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) |
|
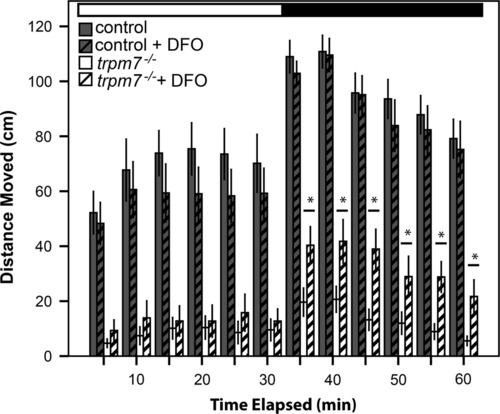
DFO partially rescues hypomotility of trpm7 mutants. DFO treatment increased spontaneous motility of 5-dpf, trpm7b508 mutant larvae. Automated analysis of motility was carried out as in Fig. 2. For each experiment, half of a single clutch of larvae from trpm7 heterozygous adults was treated with 50 µM DFO from 2-5 dpf. Motility of control larvae was unaffected by DFO treatment (Student′s t-test, p>0.4). Motility of the trpm7 mutant was significantly increased by treatment with DFO in darkness (Student′s t-test, p<0.05). Each group tested consisted of 23-24 animals. Error bars represent SEM. Asterisks, significantly different values. PHENOTYPE:
|
|
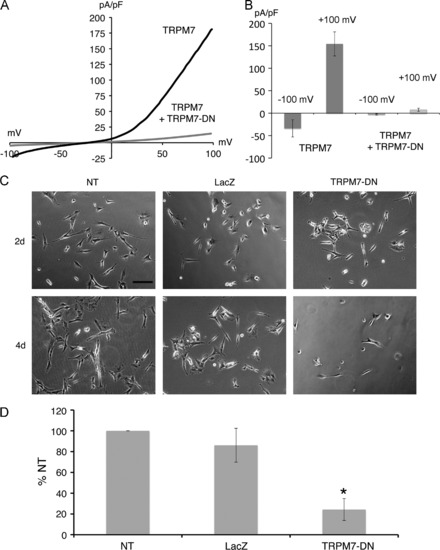
Expression of TRPM7 dominant-negative (TRPM7-DN) suppresses differentiation by causing cell death. (A) Representative traces show the current-voltage relationship in HEK-293 cells with expression of wild-type TRPM7 alone or with coexpression of wild-type TRPM7 and the TRPM7-E1047K pore mutant (TRPM7-DN). (B) Co-expression of TRPM7-DN with wild-type TRPM7 in HEK-293 cells suppressed TRPM7 current densities at 120 mV and +100 mV. (C) Representative phase-contrast images of SH-SY5Y cells treated with retinoic acid (RA), at 4 and 6 days after plating. Shown are non-transduced (NT) cells, cells transduced with LacZ for 2 and 4 days, and cells transduced with TRPM7-DN for 2 and 4 days. Scale bar=200 μm. (D) Cell viability of non-transduced (NT), LacZ expressing, and TRPM7-DN expressing SH-SY5Y cells treated with RA 6 days after plating and 3 days post-transduction. Viability of TRPM7-DN transduced and NT cells was significantly different (Student′s t-test, p=0.006). Viability was expressed as percent of non-transduced (% NT) cells. |
|
DFO does not prevent cell death in SH-SY5Y cells expressing TRPM7-DN, whether or not cells are induced to differentiate with retinoic acid. A) SH-SY5Y cells were plated in 12-well plates at a density of 4 x 104 cells/well in complete media. An adenovirus expressing the TRPM7-E1047K pore mutant (TRPM7-DN) at a multiplicity of infection (M.O.I.) of 30 was used to suppress TRPM7 channel function. To assess whether DFO is protective against TRPM7-DN-indcued cell death, the drug was co-administered at increasing concentrations (μM). Cells treated with DFO alone exhibited cell death. Non-transduced (NT) and cells transduced with an adenovirus expressing LacZ were used as negative controls. Cells were counted 72 hrs after plating and are expressed as a percentage of non-transduced cells (% NT). An adenovirus expressing GFP (AdGFP) was used to assess viral transduction efficiency at an M.O.I. of 30. Shown are relief contrast (RC) and fluorescence (FL) images of virally transduced cells. Scale bar, 200 μm. B) SH-SY5Y cells were plated in 12-well plates at a density of 4 x 104 cells/well in complete medium. SH-SY5Y cells were induced to differentiate by culturing in complete medium with 10 μM retinoic acid (RA) for 72 hrs. After 72 hrs of differentiation, cells were transduced with TRPM7-DN at a M.O.I. of 30, and DFO was co-administered at increasing concentrations (μM). Cells treated with DFO alone exhibited cell death. Non-transduced cells and cells transduced with an adenovirus expressing LacZ were used as negative controls. Cells were counted 6 days after plating. |
Reprinted from Developmental Biology, 386(2), Decker, A.R., McNeill, M.S., Lambert, A.M., Overton, J.D., Chen, Y.C., Lorca, R.A., Johnson, N.A., Brockerhoff, S.E., Mohapatra, D.P., Macarthur, H., Panula, P., Masino, M.A., Runnels, L.W., and Cornell, R.A., Abnormal differentiation of dopaminergic neurons in zebrafish trpm7 mutant larvae impairs development of the motor pattern, 428-39, Copyright (2014) with permission from Elsevier. Full text @ Dev. Biol.