- Title
-
Post-transcriptional mechanisms contribute to Etv2 repression during vascular development
- Authors
- Moore, J.C., Sheppard, S., Shestopalov, I.A., Chen, J.K., and Lawson, N.
- Source
- Full text @ Dev. Biol.
|
Etv2 is down-regulated during vascular development. (A) Graph of nCounter quantification for etv2, fli1a, fli1b, and kdrl at the indicated developmental stages. Values are normalized to actb2 (beta-actin) and eef1a1l1(ef1alpha). (B, D) Whole mount in situ hybridization using an antisense etv2 riboprobe at 5ss and 18ss. (C, E) Embryos at 5 ss and 18 ss immunostained with Etv2 antibody and anti-rabbit Alexa-488. (B, C) Dorsal views of flat-mounted embryos, anterior to the left. (D, E) Lateral views, anterior to the left. (F–I) Two-photon micrographs of trunk vessels in fixed Tg(fli1a:negfp)y7 embryos immunostained with antibodies against (F, G) Etv2 or (H, I) Fli1b. Left panels, immunostained protein detected with Alexa-568 secondary antibody. Middle panels, transgenic expression of nuclear localized EGFP. Right panels, overlay of Alexa-568 and EGFP signals. Embryos at (F, H) 25 hpf or (G, I) 48 hpf. |
|
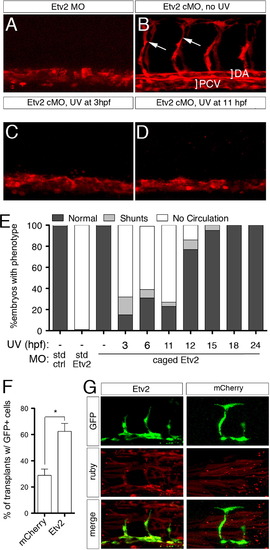
Etv2 is required only during early stages of vascular development. (A–D) Confocal images of trunk blood vessels in Tg(fli1a.ep:DsRedex)um13 embryos at 30 hpf. Lateral views, dorsal is up, anterior to the left. Embryos injected with (A) 5 ng standard Etv2 Morpholino (MO) or (B) 2 ng Etv2 caged MO (cMO), but not illuminated with UV light. ISVs (arrows), dorsal aorta (DA; bracket) and posterior cardinal vein (PCV; bracket) are indicated. (C, D) Embryos injected with Etv2 cMO exposed to UV light at (C) 3 hpf or (D) 11 hpf. (E) Penetrance of indicated circulatory defects in embryos at 48 hpf following injection with MO and UV exposure as indicated. (F) Percentage of mosaic miniRuby-positive host embryos showing successful transplantation of Tg(fli1:EGFP)y1 donor cells. Donor embryos were injected with 100 pg of mcherry or etv2 mRNA. *p<0.05. (G) Representative confocal images of wild type hosts with contribution to both vascular (green) and non-vascular (red) tissue; donor embryos were injected with mRNA encoding either Etv2 or mCherry. |
|
The etv2 3′UTR represses a heterologous reporter in endothelial cells. (A, B) Representative confocal micrographs of 48 hpf wild type embryos co-injected with 25 pg of transposase mRNA and 25 pg of a Tol2 bis-cistronic endothelial cell autonomous sensor construct encoding mCherry fused to a (A) control 3′UTR or the (B) EST etv2 3′UTR sensor. (Top) Endothelial expression of the control EGFP transgene. (Middle) Endothelial expression of the mCherry sensor transgene. (Bottom) Merge of green and red channels. Lateral views, dorsal is up, anterior to the left. (C) Quantification of relative mCherry fluorescence levels compared to EGFP following 3′ UTR sensor injection. *p<0.05, N. S.=Not significant. |
|
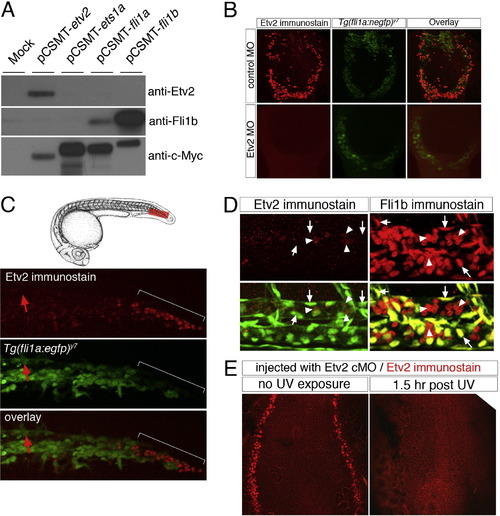
Etv2 protein expression. (A) SDS–PAGE gel of HEK293T lysates transfected with CMV-driven expression vectors encoding myc-tagged zebrafish Etv2, Ets1a, Fli1a, Fli1b, or left untransfected (mock). Lysates from each sample were run on triplicate immunoblots, which were individually probed with Etv2 or Fli1b polyclonal antiserum, or a monoclonal against the myc-epitope (9E10). The Etv2 polyclonal serum recognizes a single band that is the same size as that recognized following immunodetection for the myc epitope. (B) Tg(fli1a:negfp)y7 embryos at 18 hpf injected with 5 ng of control or Etv2 MO followed by immunostaining using Etv2 polyclonal serum and Alexa-568 secondary. Etv2 antibody staining is clearly visible in embryos injected with control MO, but absent in embryos injected with 5 ng of an Etv2 translation blocking morpholino. (C) Top, camera lucida drawings of embryo at approximately 24 hpf. Bottom, immunostaining of an Tg(fli1a:egfp)y1 embryo with Etv2 polyclonal serum and alexa-568 secondary at 24 hpf. Faint Etv2 expression can be observed in many EGFP-positive cells within the caudal vein plexus, while strong Etv2 expression is apparent in a separate EGFP-negative population of cells (indicated by a white bracket). Etv2 expression is not detectable in the caudal aorta at this time point (red arrows). (D) Two photon micrographs of Tg(fli1a:negfp)y7 embryos immunostained with Etv2 (left panels) or Fli1b (right panels) polyclonal serum. Top panels are signal from Alexa-568 secondary antibody. Bottom panels are overlay of Alexa-568 and EGFP fluorescence. Images are higher magnification views of embryos shown in Fig. 1 G and I. Left, arrows indicate EGFP-positive endothelial nuclei that do not express Etv2; arrowheads indicate EGFP-negative/Etv2-positive cells within the dorsal aorta. Right, arrows indicate EGFP-positive endothelial nuclei that also express Fli1b; arrowheads denote EGFP-negative/Fli1b-positive blood cells circulating within the dorsal aorta. (E) Flat mounted embryos at 5 ss immunostained with Etv2 polyclonal serum. Embryos were injected with Etv2 cMO and left in the dark (no UV exposure) or illuminated at approximately 2 ss with UV and allowed to develop for 1.5 h (1.5 h post UV) prior to fixation and immunostaining. |
|
Evidence for alternative 3′ UTRs encoded by the zebrafish etv2 locus. (A) Schematic depicting etv2 intron/exon structure and alternative 3′UTR lengths. Evidence for the existence of each isoform derived from annotation, 3′RACE, and RT-PCR is indicated. (B) 3′ RACE products amplified from 24 hpf embryos. (C, D) RT-PCR of etv2 UTRs from (C) wild type embryos at 6 to 18 ss and (D) wild type or MZDicer embryos at 24, 30, or 48 hpf. Genomic DNA (g) was used as a positive control. +denotes reverse transcribed cDNA template; indicates template without reverse transcription to rule out genomic DNA contamination. (E) Diagram of endothelial cell autonomous 3′ UTR sensor construct and experimental procedure for measuring post-transcriptional regulation of 3′ UTRs. |
|
Multiple let-7 family members can repress the etv2 3′ UTR. Embryos were co-injected with gfp-est-etv2 3′ UTR sensor (25 pg) and mcherry mRNAs (25 pg), along with indicated RNA duplexes. Bright field (left column), green fluorescent (middle column) and red fluorescent (right column) images of injected embryos were captured at 24 hpf. |
Reprinted from Developmental Biology, 384(1), Moore, J.C., Sheppard, S., Shestopalov, I.A., Chen, J.K., and Lawson, N., Post-transcriptional mechanisms contribute to Etv2 repression during vascular development, 128-40, Copyright (2013) with permission from Elsevier. Full text @ Dev. Biol.