- Title
-
Transdifferentiation of fast skeletal muscle into functional endothelium in vivo by transcription factor etv2
- Authors
- Veldman, M.B., Zhao, C., Gomez, G.A., Lindgren, A.G., Huang, H., Yang, H., Yao, S., Martin, B.L., Kimelman, D., and Lin, S.
- Source
- Full text @ PLoS Biol.
|
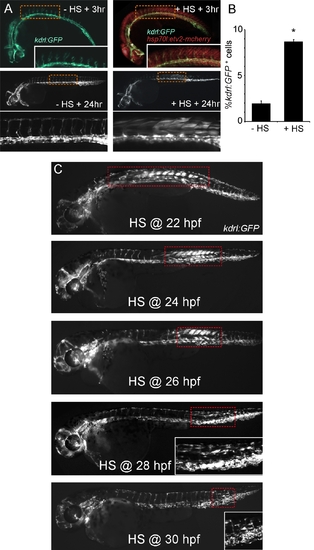
Ubiquitous Etv2 expression induces ectopic vascular gene expression in the trunk of 48 h zebrafish embryos. (A) hsp70l:etv2/kdrl:GFP embryo at 27 h postfertilization (hpf) (high magnification view of trunk inset) and 48 hpf exhibit normal vascular-specific GFP expression and no Etv2-mCherry when not treated with heat shock (HS+3 h and +24 h). Embryos heat shocked at 24 hpf exhibit normal vascular GFP expression and strong ubiquitous nuclear Etv2-mCherry expression at 27 hpf (+HS+3 h). By 24 h post–heat shock (48 hpf) robust ectopic GFP expression is present in the trunk. (B) Flow cytometric analysis of single cells isolated from three separate clutches of hsp70l:etv2/kdrl:GFP embryos treated with or without heat shock at 24 hpf and analyzed at 48 hpf. Ectopic Etv2 expression causes the percentage of GFP+ cells to increase from ~2% to ~8% of the total. T-test (*) p<0.05. (C) Response to Etv2 overexpression is developmentally restricted. Hsp70l:etv2/kdrl:GFP embryos heat shocked (HS) at 22, 24, 26, 28, or 30 hpf exhibit decreasing numbers of GFP+ cells and a shift from anterior trunk to tail. Heat shock after 30 hpf did not cause ectopic GFP+ cells. Dashed boxes highlight the Etv2 responsive cell populations. |
|
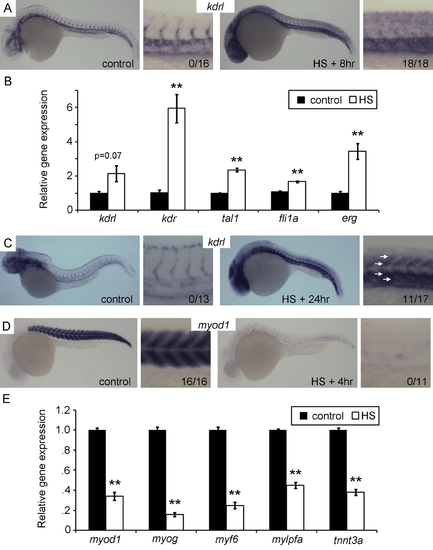
Etv2 overexpression induces vascular gene expression and represses muscle gene expression. (A and B) Vascular genes are induced 8 h post-HS. (A) In situ hybridization (ISH) for kdrl demonstrates broad ectopic expression including in the trunk following HS, but normal vascular restricted expression in control embryos. The numbers in the higher magnification views represent the number of embryos exhibiting ectopic expression over the number observed. (B) Quantitiative RT-PCR (qPCR) for kdrl, kdr, tal1, fli1a, and erg 8 h post-HS versus non–heat shocked controls. (C) ISH for kdrl at 24 h post-HS demonstrates increase expression in the trunk (white arrows). The apparent age discrepancy between the control and the HS+24 h embryo is due to developmental delay caused by Etv2 overexpression. (D and E) Muscle genes are repressed by Etv2 overexpression. (D) ISH for myod1 demonstrating near complete loss of expression 4 h post-HS. The numbers in the higher magnification views represent the number of embryos exhibiting normal expression levels. (E) qPCR for muscle genes myod1, myog, myf6, mylpfa, and tnnt3a shows significantly decreased expression 4 h post–heat shock. qPCR was performed on three separate clutches. (**) p<0.01, t test versus non–heat shocked control. |
|
Fast skeletal muscle expresses ectopic endothelial genes following Etv2 overexpression. (A) Immunostained sections through the trunk of 48 hpf hsp70l:etv2/fli1a:EGFP embryos that were untreated (control) or heat shocked at 24 hpf (HS+24 h). Sections were stained for GFP and fast muscle myosin. Nuclei are stained with DAPI in the mergeDAPI panels. fli1a:EGFP is normally expressed in the intersomitic vessels (ISVs) and axial vessels (AVs) of control sections. However, following heat shock, many fast muscle myosin positive cells were also GFP positive (A). ROI is the region of interest highlighted by the dashed box in each panel. One section from 20 different embryos was observed for each treatment group with similar results within each group. (B) Confocal projection images of a kdrl:GFP+ and mylpfa:mRFP+ double positive muscle fiber (arrow) in a living embryo 12 h post–heat shock. (C) Time lapse imaging of the trunk (left column) and at the single cell level (right column) of a mylpfa:mRFP/hsp70l:etv2/kdrl:GFP triple transgenic embryo beginning at 8 h post–heat shock (t0+8 h). Heat shock occurred at 24 hpf. A few Etv2-mCherry+ nuclei are present in the first panel (arrowhead). The normal GFP+ intersomitic vessels (ISVs) and axial vessels (AVs) are labeled. mylpfa:mRFP labels fast muscle fibers in red. In the trunk, GFP expression first appears in muscle fibers between t0+8 h to t0+10 h and progresses in an caudal to rostral wave. mRFP+ fibers induce GFP expression and then soon switch off mRFP expression. ISV sprouts appear to apoptose and regress (asterisks). At the single cell level, mRFP+ fibers become GFP+ and then change morphology, a single cell is highlighted by a dashed outline in the right column. |
|
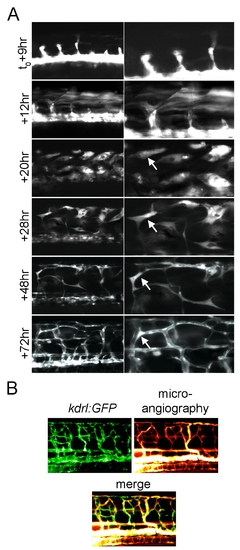
Fast skeletal muscle converts to functional endothelial cells following Etv2 expression. (A) Extended time lapse imaging of the trunk of a kdrl:GFP/hsp70l:etv2 embryo showing ectopic GFP expressing cells change morphology from fiber-like (+12 h) to spindle-like (+20 h, +28 h) to form a network of thin cells (+48 h) and finally appear to form lumenized vessels (+72 h). The two panels shown at each time point are two magnifications of the same image. (B) Microangiography demonstrates the newly formed vessels are functional. Rhodamine dextran dye was injected into the circulation of a 4 dpf (+72 h post–heat shock) kdrl:GFP/hsp70l:etv2 embryo. Rhodamine labels within all of the newly formed vessels and no vascular leakage are observed. |
|
Etv2 cell autonomously initiates transdifferentiation of muscle cells. (A) Blastula cell transplantation was performed from triple transgenic, mylpfa:mRFP/hsp70l:etv2/kdrl:GFP+, into wild-type embryos. Approximately 10 cells were transplanted per embryo. Transplanted embryos were raised until 22 hpf, at which point they were selected for embryos displaying mylpfa:mRFP expression in distinct regions absent in kdrl:GFP, region of interest (ROI) boxed in (B) corresponds to images in (C). These embryos were then either heat shocked or left as no heat shock controls. Embryos were then analyzed for mylpfa:mRFP/kdrl:GFP coexpression at 10 h post–heat shock and followed out to 42 h post–heat shock (C). A muscle cell labeled with the arrow undergoes transdifferentiation to form a lumenized vessel (C). (D) Quantification of transdifferentiation efficiency per muscle cell. Only clearly distinguishable muscle cells were counted. Thirty-eight chimeric embryos, 312 total cells, were observed in the heat-shocked condition, and 20 chimeric embryos, 143 total cells, were observed for the control non–heat shocked condition. |
|
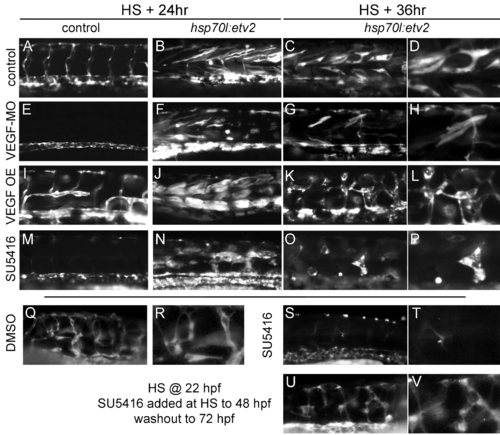
VEGF signaling is dispensable for induction but necessary for maturation of Etv2 induced vasculature. (A–D) Control kdrl:GFP embryos (A) or kdrl:GFP/hsp70l:etv2 (B–D) embryos following heat shock at 24 hpf and imaged at 24 h or 36 h post–heat shock. Control embryos exhibit normal vascular kdrl:GFP expression (A), while kdrl:GFP/hsp70l:etv2 embryos exhibit the ectopic GFP and morphological changes previously described (B–D). (E–H) VEGFAa morpholino (VEGF-MO) treated embryos lack intersomitic vessels (E) but still induce kdrl:GFP in muscle fibers (F). However, kdrl:GFP+ muscle fibers do not undergo the normally observed morphological changes following heat shock–induced expression of Etv2 (G,H). (I–L) Overexpression of VEGFAa121 (VEGF OE) driven by the hsp70l promoter results in disorganization and expansion of the normal vasculature (I). Following heat shock–induced expression of Etv2, no significant change in the number of muscle fibers expressing kdrl:GFP is observed (J). However, the morphological changes observed are accelerated in the presence of elevated VEGF (K,L). (M–P) Treatment of embryos with SU5416, a Kdr inhibitor, similarly inhibits intersomitic vessel development in control embryos (M). However, drug treatment does not inhibit induction of kdrl:GFP following heat shock–induced Etv2 expression in muscle (N). The morphology and survival of these fibers is compromised when Kdr is inhibited (O,P). (Q–V) Removal of VEGF inhibitor SU5416 24 h following heat shock allows for survival and maturation of transdifferentiated cells. Kdrl:GFP/hsp07l:etv2 embryos were heat shocked at 22 hpf and then treated with DMSO carrier or SU5416 for 24 h at which point the drug was either maintained (S,T) or removed (U,V) and the embryos were allowed to develop until 72 hpf. (Q,R) DMSO controls exhibit a transdifferentiated vascular network similar to that in untreated controls. (S,T) Sustained SU5416 treatment largely abolishes the kdrl:GFP+ vascular network. (U,V) Removal of SU5416 24 h post–heat shock results in the development of a vascular network similar to controls (Q,R), suggesting VEGF signaling modulates the survival and maturation of muscle-derived vessels and not the initial induction. For all experiments at least 20 embryos were observed with similar results. |
|
Canonical Wnt signaling is necessary for Etv2-induced transdifferentiation of muscle fibers. (A) Inhibition of Wnt signaling using XAV939 or heat shock–inducible, dominant negative Tcf3 (hsp70l:tcfΔC-EGFP) blocks Etv2-induced kdrl:GFP expression. However, activation of Wnt signaling using CHIR99021 or heat shock–inducible, constitutively active β-catenin (hsp70l:caβ-catenin-2A-TFP) also blocks Etv2-induced kdrl:GFP expression. (B) XAV939 dose dependently inhibits Etv2-induced kdrl:GFP expression. Kdrl:GFP expression was quantified by counting the number of GFP+ myotomes with a myotome being considered positive if >3 muscle fibers within a given myotome were GFP+. (C) CHIR99021 dose dependently inhibits Etv2-induced kdrl:GFP expression. (D) XAV939 (40 μM) inhibits Etv2-induced kdrl:GFP expression independent of heat shock time, while CHIR99021 (50 µM) inhibits at 22 and 24 h heat shocks, has no effect at 26 h heat shock, but enhances Etv2-induced kdrl:GFP expression at 28 h heat shock. For all data t test was used for statistical comparisons with (*) p<0.05 and (**) p<0.01. |
|
A tightly controlled level of Wnt signaling is necessary for muscle to transdifferentiate into endothelial cells. (A and B) Dose-dependent rescue of XAV939 inhibition of kdrl:GFP expression by the addition of Wnt activator CHIR99021. (A) Images of the trunk of 48 hpf embryos following treatment with the noted compounds and heat shock at 24 hpf. Note that 40 μM XAV939 alone almost completely inhibits ectopic GFP expression and the addition of 5–25 μM of CHIR99021 can rescue this inhibition with 15 μM being the most affective dose. CHIR99021 doses greater than 50 μM do not rescue XAV939 inhibition. (B) Quantification of the observations in (A). t test, (*) p<0.05 comparing Control (Con) to drug treated and (#) p<0.05 comparing XAV939 (40 μM) to XAV939 (40 μM) plus varying doses of CHIR99021. (C) Schematic of muscle cell transdifferentiation into endothelial cells. Muscle cells exhibiting permissive levels of canonical Wnt activity are responsive to a pulse of Etv2 expression, resulting in repression of muscle gene expression and initiation of vascular gene expression. Cells expressing vascular genes respond to VEGF signals and change morphology from muscle fiber to thin multibranched and finally to lumenized patent endothelium. |
|
Hsp70l:etv2 is as effective as etv2 mRNA injection at inducing kdrl:GFP expression. (A) A shield stage embryo that was heat shocked at dome stage and imaged for mCherry expression. Nuclear-localized Etv2-mCherry is visible. (B) Heat shock of hsp70l:etv2-mCherry transgenic embryos at dome stage induces kdrl:GFP expression at tailbud stage similar to that seen with mRNA injection. (C) mRNA injection of etv2 induces kdrl:GFP expression at tailbud stage. The embryos overexpressing Etv2 in (C) and (D) gastrulated abnormally due to overexpressed Etv2. |
|
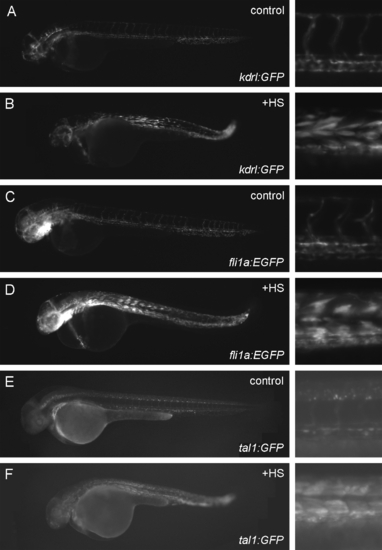
Kdrl:GFP, fli1a:EGFP and tal1:GFP are all induced in the trunk of hsp70l:etv2 embryos following heat shock. Hsp70l:etv2 was crossed into kdrl:GFP (A, B), fli1a:EGFP (C, D), or tal1:GFP (E, F), and the resulting embryos were either left at control temperature or were heat shocked at 22 hpf and then imaged at 48 hpf. Control embryos never exhibited ectopic GFP expression in any group. Heat-shocked embryos (+HS) always exhibited ectopic GFP expression in the trunk (right column is high magnification image of the trunk corresponding to the adjacent embryo in the left column), although tal1:GFP was significantly weaker than the other two transgenes. At least 20 embryos were observed for each treatment with similar results. |
|
ISH of vascular genes following Etv2 expression. Fli1a (A), tal1 (B), erg (C), and kdr (D) are all induced in the trunk of embryos 8 h post–heat shock (HS+8 h). Normally these genes are specifically expressed in the vasculature at this time point (control), although tal1 is more strongly expressed in the blood and neurons in the CNS. Note that fli1a and erg have almost ubiquitous expression at this time point, while tal1 and kdr are more restricted to ectopic expression in the trunk. Quantification of the number of embryos demonstrating ectopic expression over the number observed is in the bottom right corner of the corresponding high-magnification trunk image for each group. Eight hours post–heat shock was chosen because it is the time when ectopic expression of kdrl:GFP was first noted in our transgenic analysis. |
|
ISH of muscle genes following Etv2 expression. Myog (A), myf6 (B), mylpfa (C), and tnnt3a (D) are all repressed in the trunk of embryos 4 h post–heat shock (HS+4 h). Normally these genes are strongly and specifically expressed in the musculature at this time point (control). Expression of myog and myf6 is almost completely abolished (A, B). Mylpfa and Tnnt3a are reduced but much less so (C, D). Quantification of the number of embryos demonstrating normal muscle expression over the number observed is in the bottom right corner of the corresponding high-magnification trunk image for each group. Four hours post–heat shock was chosen since it is the peak of heat shock–induced Etv2 expression. |
|
Fli1a and Tal1 overexpression at 24 hpf is not sufficient to induce ectopic kdrl:GFP expression at 48 hpf. (A) Heat shock–induced etv2, fli1a, or tal1 are all capable of inducing ectopic kdrl:GFP+ cells in the early embryo. Kdrl:GFP embryos were injected with the indicated heat shock–inducible transgenes, heat shocked at shield stage, and imaged at 16 somite stage. Nuclear mCherry (NLS-mCherry) was not able to induce ectopic kdrl:GFP while etv2, fli1a, and tal1 were (bracketed area in lateral view of embryo). The number of embryos exhibiting ectopic GFP expression over the total number observed is represented in the top right corner of each panel. (B) Kdrl:GFP transgenic embryos were injected with hsp70l:etv2, hsp70:fli1a, or hsp70:tal1 transgenes and heat shocked at 24 hpf. Each transcription factor was labeled with mCherry and expression was confirmed by imaging 3 h post–heat shock (inset). GFP expression was imaged at 48 hpf. Etv2 overexpression resulted in strong ectopic GFP expression, but neither Fli1a nor Tal1 was sufficient for inducing the same response. The ratio in the bottom right corner of each panel represents the GFP positive embryos over the total observed. |
|
Slow muscle fibers do not respond to Etv2 overexpression. Immunostained sections through the trunk of 48 hpf hsp70l:etv2/fli1a:EGFP embryos that were untreated (control) or heat shocked at 24 hpf (HS+24 h). Sections were stained for GFP and slow muscle myosin. Nuclei are stained with DAPI in the mergeDAPI panels. fli1a:EGFP is normally expressed in the intersomitic vessels (ISVs) and axial vessels (AVs) of control sections. No co-staining of GFP and slow muscle myosin was observed (arrows). ROI is the region of interest highlighted by the dashed box in each panel. One section from 20 different embryos was observed for each treatment group with similar results within each group. |
|
Fast muscle–specific mylpfa:mRFP is co-expressed with kdrl:GFP following overexpression of Etv2. (A) Confocal image of the trunk of a kdrl:GFP/mylpfa:mRFP/hsp70l:etv2 triple transgenic embryo heat shocked at 24 hpf and imaged at 12 h post–heat shock. A GFP/mRFP double positive muscle fiber is highlighted by the arrow and x-axis and y-axis z-plane projections are presented below and to the right of the image respectively. (B) Fast muscle myosin and GFP colocalize in the trunk of kdrl:GFP/hsp70l:etv2 transgenic fish heat shocked at 24 hpf and imaged at 48 hpf. Red muscle fibers colocalize with GFP (white arrows). A strongly GFP positive cell located where a muscle fiber normally would be is negative for fast muscle myosin (large white arrowhead), suggesting this cell has lost its muscle cell identity. |
|
Etv2 overexpression is toxic to angiogenic sprouts and its maintained expression prevents kdrl:GFP expression. (A) Time lapse imaging of an intersegmental vessel angiogenic sprout from Figure 3C showing apparent apoptosis. (B) Multiple heat shocks of hsp70l:etv2 embryos prevent ectopic kdrl:GFP expression. Embryos were initially heat shocked at 24 hpf and then either maintained at normal temperatures or were treated with heat shock every 12 h the indicated number of times. Embryos treated with multiple heat shocks displayed abnormal morphology whose severity correlated with the number of heat shocks, including cardiac edema suggestive of circulatory failure (left column and inset). Control embryos heat shocked four times appeared similar to non–heat shocked embryos, HS(–). When Etv2 expression was maintained for the whole period (4× heat shocks), kdrl:GFP was not ectopically induced and was reduced or absent in the normal vasculature (right column). |
|
Lineage tracing of muscle cells demonstrate they are a source of new blood vessels following Etv2 expression. Lineage tracing of fast muscle fibers in mylpfa:cre-ERt2 injected kdrl:GFP/hsp70l:etv2/ubi:Switch fish demonstrates endothelial cells derived from muscle fibers (arrow). The ubi:Switch transgene changes from GFP to mCherry following recombination initiated by Cre. The mylpfa promoter specifically drives Cre expression in fast muscle fibers. In non–heat shocked embryos (HS) only mCherry muscle fibers are observed (n = 13), while following heat shock mCherry+, kdrl:GFP+ vessels were observed in 5 out of 15 embryos observed. Note that the kdrl:GFP transgene in the vasculature is significantly brighter than the ubi:Switch GFP+ background. All embryos were treated with hydroxytamoxifen (5 μM) immediately after heat shock or the equivalent time for non–heat shocked controls. |
|
Etv2 cell autonomously initiates transdifferentiation of muscle cells. Blastula cell transplantation was performed from triple transgenic, mylpfa:mRFP/hsp70l:etv2/kdrl:GFP+, into wild-type embryos. Approximately 10 cells were transplanted per embryo. Transplanted embryos were raised until 22 hpf at which point they were selected for embryos displaying mylpfa:mRFP expression in distinct regions absent in kdrl:GFP. These embryos were then either heat shocked or left as no heat shock controls. Embryos were then analyzed for mylpfa:mRFP/kdrl:GFP coexpression at 10 h post–heat shock and followed out to 44 h post–heat shock. Two kdrl:GFP positive muscle fibers, one still mylpfa:mRFP positive (arrow), under-go transdifferentiation to form functional vessels supporting blood cell flow (see Movie S4). |
|
Etv2 induces vascular marker gene expression in C2C12 cells but not in other cell types. (A) Induction of endothelial cells marker expression in myoblast cell line C2C12. Fli1, Scl, Kdr, and vascular endothelial cadherin (Vecad) expression levels detected by qPCR were compared between Etsrp/Etv2 transfection and control mCherry transfection. Fold of induction compared to nontransfected cells is shown. (B) PCR detection of vascular marker expression in three tumor cell lines that were transfected with Etv2. No induction of the listed endothelial cell markers was detected. |