- Title
-
Distinct Functional and Temporal Requirements for Zebrafish Hdac1 during Neural Crest-Derived Craniofacial and Peripheral Neuron Development
- Authors
- Ignatius, M.S., Unal Eroglu, A., Malireddy, S., Gallagher, G., Nambiar, R.M., and Henion, P.D.
- Source
- Full text @ PLoS One
|
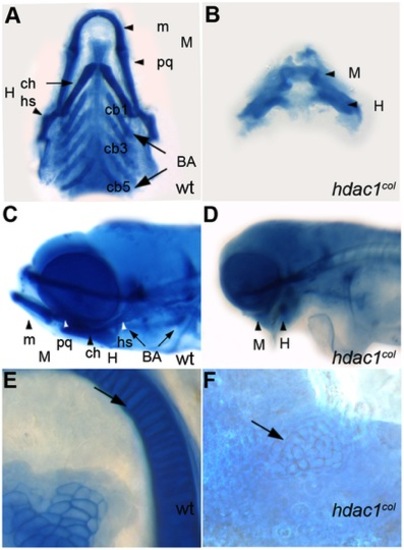
Craniofacial defects in hdac1b382 mutants. A–E alcian blue stained jaw elements in 5 dpf wild type A, C, E and hdac1b382 mutants B, D, F; A, B Ventral view of dissected craniofacial cartilages of wild-type and hdac1b382 mutant; C, D lateral view of head region in wild-type and hdac1b382 mutant; E,F, High magnification of the mandibular chondrocytes (arrows) in wild-type and hdac1b382 mutant; m, meckels; pq, platoquadrate; M, mandibular; ch, ceratohyal; hs, hyosymplectic; H, hyoid; cb1-5, ceratobrachials 1-5; BA, branchial arches. PHENOTYPE:
|
|
Craniofacial progenitor differentiation defects in the mandibular and hyoid arches of hdac1b382mutants. A, C, E, G wild-type, B, D, F, H hdac1b382 mutants; A, B Lateral views of the head with dlx2 expression labeling developing jaw elements in 48 hpf embryos. C, D, Lateral views of the head with dlx3 expression labeling developing jaw elements in 48 hpf embryos. E, F ventral views of the head in 68 hpf embryos expressing col2a1 in different jaw structures, col2a1. G, H ventral views of the head of 68 hpf embryos expressing sox9a in different jaw elements. M, mandibular; H, hyoid; BA, branchial arches. |
|
Neural crest-derived posterior branchial arch progenitor specification is defective in hdac1b382 mutants. A, C, E, G, I, K wild-type embryos, B, D, F, H, J, L hdac1b382 mutants. A, B Dorsal view of embryos at 25 hpf with dlx2 expression labeling the mandibular, hyoid and branchial arch precursor populations. C, D Lateral views of the head region at 25 hpf of crestin expression in the head region, black arrowheads indicate branchial arch populations. E, F, Lateral views of the head region at 25 hpf of hoxb3a expression in the hind-brain and branchial arch precursors. G, H, Lateral views of the head region at 96 hpf of col2a1 expression in mandibular, hyoid and branchial arches. I, J, Lateral views of the head region at 56 hpf of TUNEL-positive staining in the head and jaw regions. K, L, Side views of the head region at 32 hpf with tbx1 expression highlighting the endodermal pouches. M, mandibular; H, hyoid; BA, branchial arches. |
|
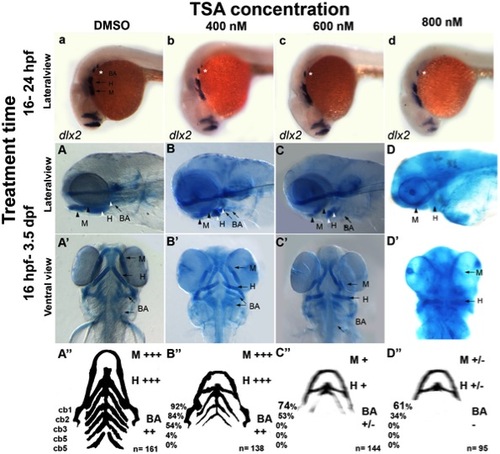
Treatment with the HDAC inhibitor TSA can reproduce the hdac1b382 mutant phenotype. A–d Lateral views of wild-type embryos treated with DMSO, 400 nM, 600 nM and 800 nM TSA from 16–24 hpf after which embryos were fixed and stained for dlx2 expression. A–D and A′–D′ Alcian blue stained 3.5 dpf wild-type embryos under different TSA treatment conditions, all embryos were treated between 16 hpf and 3.5 dpf after which embryos were fixed and then stained with alcian blue; A–A′ DMSO controls, B–B′ 400 nM TSA, C–C′ 600 nM TSA, D–D′ 800 nM TSA. A–D lateral view; A′–D′ ventral views. A′′–D′′ schematic with summary of craniofacial defects at different TSA treatment conditions. M, mandibular; H, hyoid; cb1-5, cerato-branchials 1-5; BA, branchial arches,+++ wild-type,++ reduced in size compared to wild-type,+severely reduced compared to wild-type, +/ severely reduced or absent altogether. EXPRESSION / LABELING:
PHENOTYPE:
|
|
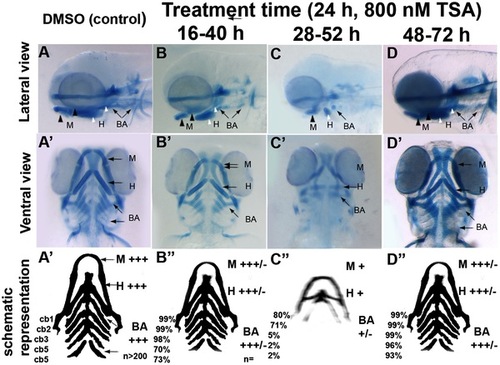
Differential temporal requirements for HDAC function during craniofacial development. A–D and A′–D′ Wild-type embryos treated with TSA for 24 hpf at different stages of embryonic development. After treatment periods other than 48–72hpf, embryos were washed to remove TSA and then allowed to develop until 3.5 dpf. Embryos were fixed at 3.5 dpf and then stained with alcian blue. A–D lateral views, A′-D′ ventral views. A–A′ are DMSO-treated controls, B–B′ 16–40 hpf TSA-treated embryos, C–C′ 28–52 hpf TSA-treated embryos, D-D′ 48–72 hpf TSA-treated embryos. A′′-D′′ schematic with summary of craniofacial defects at different TSA treatment concentrations. M, mandibular; H, hyoid; cb1-5, cerato-branchials 1-5; BA, branchial arches;+++ wild-type,++ reduced in size compared to wild-type,+Severely reduced compared to wild-type, +/ severely reduced or absent altogether. PHENOTYPE:
|
|
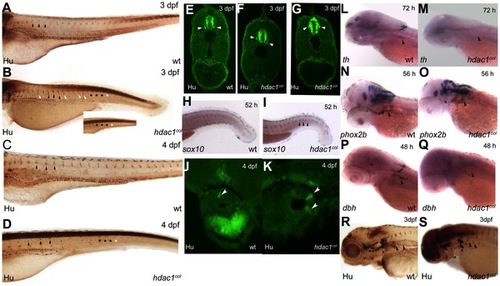
Sensory, enteric and sympathetic neuron development in hdac1b382 mutants. A–D Anti-Hu/16A11 staining of wild-type and hdac1b382 mutants at 3dpf and 4dpf. Black arrowheads indicate normally positioned DRG in wild-type and hdac1b382 mutants. White arrowheads indicate examples of DRG neurons in ectopic locations. Black asterisks (A-D and inset) indicate the regions in the tail of wild-type and hdac1b382 mutants where DRG are present. White asterisks (B and inset, D) indicate the regions in the tail of hdac1b382 mutants where DRG are absent. E-G a cross sections of wild-type (E) and examples of 2 sections (F, G) of hdac1b382 mutants illustrating that DRG neurons in mutant embryos are often ectopically localized. H, I wild-type and hdac1b382 mutants with sox10 expression in the tail region at 52 hpf, black arrowheads in I indicate distinct foci of sox10 expression in mutant in locations where DRG would normally reside. J, K cross-sections of the anterior gut in wild-type and hdac1b382 mutants stained with anti-Hu/16A11 labeling enteric neurons at 4 dpf (white arrowheads). L, M th expression in the cervical sympathetic ganglion (black arrowhead) in wild-type and hdac1b382 mutants at 72 hpf. N, O, phox2b labeling of sympathetic neuron precursors (black arrowheads) in wild-type and hdac1b382 mutants at 56 hpf. P, Q, dbh staining of sympathetic neurons (black arrowheads) in wild-type and hdac1b382 mutants at 48 hpf. R, S, Anti Hu/16A11 staining of wild-type and hdac1b382 mutants at 3dpf with black arrowheads indicating sympathetic neurons. PHENOTYPE:
|
|
Effect of HDAC inhibition on sympathetic neuron differentiation is reversible. A, B wild-type embryos treated with DMSO (A) and TSA (B) continuously from 28–52 hpf and then fixed at 52 hpf and stained for th expression, black arrowheads indicate sympathetic neurons. C–E wild-type embryos treated under three different conditions, C with DMSO between 28–72 hpf, D with TSA between 28–72 hpf, and E with TSA between 28–52 hpf after which the TSA is washed out and then the embryos are treated in DMSO between 52–72 hpf. All embryos were fixed at 72 hpf and then stained for th expression. Black arrowheads indicate th-xpressing sympathetic neurons, or their absence. EXPRESSION / LABELING:
|