- Title
-
Zebrafish Zic2a and Zic2b regulate neural crest and craniofacial development
- Authors
- Teslaa, J.J., Keller, A.N., Nyholm, M.K., and Grinblat, Y.
- Source
- Full text @ Dev. Biol.
|
Zic2 is required for craniofacial development. Alcian blue-stained cartilage in zic2 morphants at 5 dpf. (A,D) Control morphants have wild-type pharyngeal and neurocranial cartilages (53/55, 6 exp.). (B) Zic2a morphants have variable craniofacial defects, including hypoplastic pharyngeal cartilages and an incorrectly angled hyoid arch (16/54, 5 exp.; outline in 1B′). (E) Severely affected zic2a morphants have more dramatic hypoplasia (10/54, 5 exp.) or ablation of anterior cartilages (11/54, not shown). (C,F) Zic2b morphants are either wild-type (45/70, 6 exp.) or develop shortened mandibular and hyoid arches (25/70, 6 exp.). (G) Penetrance of craniofacial defects in control and morphant embryos. (A–C) are dorsal views with anterior to the left. (D–F) are lateral views. Abbreviations: cb—ceratobranchials, h—hyoid, m—mandibular, nc—neurocranium. PHENOTYPE:
|
|
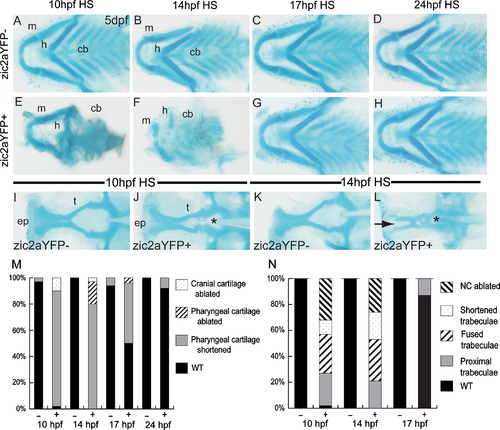
Craniofacial development is sensitive to early elevation of Zic2a levels. Alcian blue-stained cartilage in heat-shocked Tg(hsp70l:Gal4VP16);Tg(11XUAS:zic2aYFP) embryos at 5 dpf. (A,E) Heat shock (HS)-induced misexpression of Zic2aYFP at 10 hpf has a variable effect on craniofacial cartilage, ranging from shortened mandibular and hyoid arches (45/51, 5 exp., shown in 2E) to absence of all anterior cartilages (5/51, 5 exp.). (B,F) Zic2aYFP induction at 14 hpf has a similar effect, causing hypoplastic pharyngeal cartilages (29/36, 3 exp., shown in 2F) or ablation (7/36, 3 exp.). (C,G) Zic2aYFP induction at 17 hpf causes less penetrant pharyngeal cartilage hypoplasia in overexpressors (12/24, 2 exp.). (D,H) Zic2aYFP induction at 24 hpf has no effect on craniofacial cartilage (57/62, 4 exp.). (I,J) 10 hpf HS causes a narrowing of the space between trabecular cartilages (13/53, 4 exp., asterisk in 2J) or complete fusion (16/53, 4 exp.). (K,L) Zic2aYPF induction at 14 hpf causes fusion of the trabeculae (asterisk in 2L) and absence of the ethmoid plate (10/19, 1 exp., arrow in 2L), or ablation of the entire anterior neurocranium (5/19, 1 exp.). (M,N) Penetrance of cartilage defects following Zic2aYFP induction at specified timepoints. (A–H) are dissected pharyngeal cartilages, dorsal views, anterior to the left. (I–L) are dissected neurocranial cartilages, dorsal views, anterior to the left. Abbreviations: cb—ceratobranchials, ep—ethmoid plate, h—hyoid arch, m—mandibular arch, t—trabecular cartilages. PHENOTYPE:
|
|
Zic2a regulates patterning of anterior chondrogenic condensations. 2 dpf zic2a morphants (A–F) and overexpressors (G,H) stained by ISH for col2a1a or sox9a. (A,B) Sox9a expression is reduced in the posterior PAs of Zic2a-depleted embryos (18/20, 1 exp.). (C,D) Zic2a morphants have shortened trabeculae (18/20, 1 exp.) and lack the medial ethmoid plate (12/20, 1 exp., arrow in 3C). (E,F) Expression of col2a1a reiterates the shortened trabeculae and lack of ethmoid plate in Zic2a-depleted embryos (43/43, 2 exp). (G,H) Col2a1a expression in wild-type siblings shows the bilateral trabeculae and ethmoid plate (67/107, 2 exp.). (H) The ethmoid plate is ablated and trabeculae are shortened by Zic2aYFP misexpression induced at 10 hpf (38/67, 2 exp). (A,B) are lateral views. (C–H) are ventral views, anterior to the left. |
|
Zic2b promotes timely neural crest induction. (A–L) Wild-type embryos stained by two-color ISH for expression of dlx3b, foxd3, zic2a and zic2b. (A,B) At 70% epiboly, weak expression of dlx3b (orange) and zic2a (purple) form two adjacent domains. (C,D) These domains are stronger by 90% epiboly but continue to overlap, suggesting the neural plate border is not completely formed. (E,F) At 1S, dlx3b (orange) and zic2a (purple) begin to separate. (G,H) Foxd3 (orange) straddles the border beginning at 1S, overlapping neural and non-neural (dlx3b, purple) markers. (I) At 1S, NC marker foxd3 (orange) and zic2b (purple) overlap at the neural plate border. (J,K) From 3-6S, zic2b (orange) is expressed in two anterior, bilateral domains from which zic2a (purple) is excluded. (L) Zic2b expression at the neural plate border extends into the posterior neural keel. (M,N) Sna1b expression is unchanged in zic2a morphants during early somitogenesis (37/48, 5 exp.). (O) Zic2b morphants have reduced (19/47, 5 exp.) or absent (16/47, 5 exp.) NC domains. (P) The sna1b domain is reduced (11/38, 5 exp.) or more frequently absent (24/38, 5 exp.) in double zic2 morphants. (A,C,E and G) are lateral views, anterior to the right. (B, D, F and H) are high magnification images of (A, C, E and G), respectively. (I–P) are dorsal views with anterior to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Zic2a and zic2b regulate neural crest migratory onset. Morphant (A–D) or overexpressing embryos (E–F) stained by ISH for sox10. (A) At 18S, sox10 is expressed in NC cells, which have exited the neural tube in control morphants (17/21, 3 experiments). (B,C) Single knockdown of Zic2a or Zic2b results in NC cells abnormally localized to the dorsal neural tube (8/12 zic2a morphants, 2 exp.; 14/32 zic2b morphants, 3 exp.). (D) Double Zic2 depletion causes a similar buildup of sox10-positive cells on the dorsal neural tube (7/10 embryos, 2 exp.). (E,F) Transgenic embryos were heat shocked at 10 hpf to induce Zic2aYFP expression. Embryos expressing the zic2aYFP fusion protein have wild-type localization of NC cells, but increased sox10 expression (20/30, 2 exp.). (G) Quantitative real-time PCR for sox10 in 24 hpf embryos after 10 hpf HS. Relative abundance of sox10 transcript averaged across two biological replicates. (A–F) are dorsal views, anterior to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Zic2a and zic2b promote chromatophore development. Zic2 morphant embryos stained by ISH for mitfa and gch2 at 24 hpf. (A,B) Zic2a knockdown causes a mild reduction of cranial melanophore progenitors, marked by mitfa expression (21/35, 3 exp.). (C) Zic2b knockdown dramatically reduces mitfa expression in the trunk (24/27, 3 exp.). (D) Knockdown of both Zic2 proteins reduces melanophores in both cranial and trunk regions (22/29, 3 exp.) and causes NC aggregation in the cranial region (12/24, 3 exp.). (E,F) Zic2a knockdown reduces xanthophore precursors, marked by gch2 expression, in the cranial region (13/20, 3 exp.) and trunk region (16/22, 3 exp.). (G) Zic2b knockdown reduces trunk xanthophores (27/28, 3 exp.). (H) Double Zic2 knockdown decreases gch2 at both cranial and trunk axial levels (17/20 affected, 3 exp.). (I,J) Gch2-expressing cells migrating on the midbrain are wild-type in zic2a morphants (3/3, 1 exp.). (K,L) Large aggregates of migrating NC cells are observed after Zic2b knockdown (1/3, 1 exp.; asterisks in C,G,K) and double Zic2 knockdown (2/3, 1 exp.; asterisk in 7L). (A–H) are lateral views with anterior to the left, with insets showing dorsal views of the same embryos. (I–J) are transverse sections through the midbrain. |
|
Pharyngeal arch primordia are mispatterned in response to changes in Zic2a levels. (A–H) Embryos stained by ISH for dlx2a. (A) Four streams of NC cells that populate the pharyngeal arches express dlx2a (42/44, 3 exp.). (B) The dlx2a domain is reduced in PA1 and 2 of zic2a morphants (13/30, 3 exp.). (C) Zic2b knockdown has no effect on dlx2a expression in the PAs (40/44, 3 exp.). (D) The first two pharyngeal arches are reduced in some double morphants (15/46, 3 exp.), while other embryos have a reduction of dlx2a in PA3 and PA4 (11/46, 3 exp., shown in D) or all four PAs (10/46, 3 exp.). (E,F) After HS induction at 10 hpf, some Zic2aYFP-positive embryos are wild-type (13/64 embryos, 3 exp.), some have an elongated PA1 (21/64, see arrow in F) and some have reduced dlx2a staining in the arches (28/64). (G,H) Dlx2a expression remains unchanged after Zic2aYFP induction at 14 hpf (15/17, 1 exp.). (I–L) Transplant host embryos stained for biotin and alcian blue at 4 dpf. (I,J) Transplanted control morphant cells incorporated in the pterygoid process, palatoquadrate, and ethmoid plate of control morphant embryos (see arrowheads). (K,L) Transplanted Zic2a-deficient cells incorporated into the hyoid arch and the ethmoid plate of control morphant embryos. (L) Some control morphant host embryos develop with abnormal trabecular cartilages (see asterisks). (A–H) are dorsal views with anterior to the left. I–L are dissected pharyngeal and neurocranial cartilages. (I′,J′,K′ and L′) are close-up views of (I–L). Abbreviations: ep—ethmoid plate, h—hyoid, m—mandibular, pp—pterygoid process, pq—palatoquadrate. |
|
Zebrafish Zic2 patterns the ventral forebrain primordium. Embryos stained by ISH for dlx2a, nkx2.2a, and ptch2 at 24 hpf and pitx2a at 3 dpf. (A) Dlx2a marks the telencephalon, prethalamus and hypothalamus in the developing forebrain of control morphants (42/44, 3 exp.). (B) Zic2b knockdown reduces dlx2a staining in the prethalamus (18/35, 3 exp., see arrowhead). (C) Zic2a knockdown reduces dlx2a in the prethalamus and hypothalamus, as previously described (see Sanek and Grinblat, 2008; 39/43, 3 exp.). (D) Double zic knockdown causes patterning defects similar to single Zic2a knockdown (46/46, 3 exp.). (E,F) Zic2aYFP induction at 10 hpf strongly reduces dlx2a expression in the telencephalon (arrowhead in F) and hypothalamus (61/92, 4 exp.). (G,H) Ptch2 expression is ablated in the hindbrain and spinal cord and reduced in the medial diencephalon of Zic2aYFP-expressing embryos, but maintained in the ZLI (49/57, 2 exp., see arrows in H). (I,J) Zic2aYFP induction between 10 and 11 hpf ablates nkx2.2a expression in the hindbrain (35/35 embryos, 2 exp., arrows in J). (K,L) The stomodeum of zic2a morphants is decreased in size (55/56, 4 exp.). (A–J) are lateral views, (K, L) are ventral views, anterior to the left. Abbreviations: zli—zona limitans intrathalamica. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Zic2b expression and knockdown. (A) Embryos injected with zic2b splice-blocking MO express reduced levels of full-length zic2b transcript, and several mis-spliced transcript variants. (B,D) Wild-type embryos stained by ISH show expression of zic2b in the diencephalon, midbrain and hindbrain. (C,E) Restriction of zic2b expression to the dorsal neural tube is seen in transverse sections through the midbrain. (F–H) A midbrain neurulation defect is observed in zic2b morphants (midbrain primordium located between arrows in F–H). Zic2b morphants develop with small (35/104, 6 exp., see 1H) and closed midbrain ventricles by 24 hpf (33/104, 6 exp., see 1G). B,D are lateral views, anterior to the left. C,E are transverse sections through the midbrain. F–H are dorsal views, anterior to the left. |
|
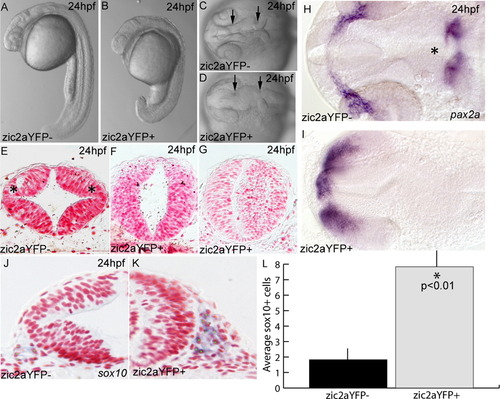
Expression of a zic2aYFP fusion protein causes developmental defects. (A–D) Embryos induced by heat shock to express the zic2aYFP fusion protein at 10 hpf have a shortened anterior–posterior axis and a characteristic kinked neural tube with a closed midbrain ventricle (located between arrows in C,D; 75/87, 4 exp.). (E) Zic2aYFP-negative siblings have distinct dorsolateral hinge points in the midbrain (asterisks in E; 2/2, 2 exp.). (F,G) Zic2aYFP induction at 10 hpf does not always preclude lumen formation, but does eliminate dorsolateral hinge points (4/4, 2 exp.). (H,I) Expression of pax2a reveals patterning defects in the retina, optic stalks and mid-hindbrain boundary of Zic2aYFP-expressing embryos (asterisk in H, 8/10, 1 exp). (J,K) Zic2aYFP-expressing embryos have more sox10-positive cells lateral to the midbrain. (L) Comparison of average numbers of sox10-positive cells in half of the neural tube in a single section (averaged from 3 randomly selected midbrain-level sections per embryo, 2 embryos per condition, 1 exp). Error bars are standard error and significance was tested with a Student′s t-test. A,B are lateral views, anterior to the left. C,D,H,I are dorsal views, anterior to the left. E–G, J, K are transverse sections through the midbrain. Green dots were added to mark cells counted as sox10-positive. |
|
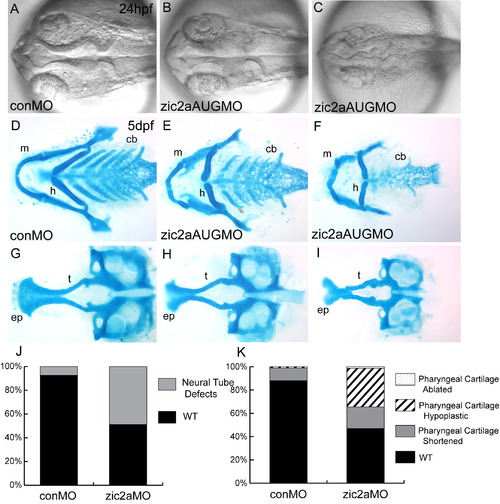
Zic2a knockdown with non-overlapping zic2a morpholinos causes craniofacial defects. (A–C) Zic2a morphants display variable neurulation defects, from wild-type (82/160, 5 exp.; shown in B) to closed or twisted midbrain ventricles (77/160, 5 exp.; shown in C). (D,G) Control morphants have mostly wild-type pharyngeal and neurocranial cartilages (242/275, 5 exp.), but a small proportion develops with mildly shortened cartilages. (E,F) Zic2a morphants display a range of craniofacial defects, including shortened pharyngeal cartilages (27/145, 5 exp.; shown in E) and hypoplastic pharyngeal cartilages with an incorrectly angled hyoid arch (48/145, 5 exp.; shown in F). (H,I) Neurocranial cartilage defects include mildly shortened and abnormally proximal trabeculae (shown in I) and correlate with the presence of pharyngeal cartilage defects. (J,K) Penetrance of neural tube defects at 24 hpf (J) and cartilage defects at 5 dpf (K) following injection of control or zic2a translation-blocking morpholino. A–C are dorsal views, anterior to the left. D–F are dissected pharyngeal cartilages, ventral views, anterior to the left. G–I are dissected neurocranial cartilages, dorsal views, anterior to the left. Abbreviations: cb—ceratobranchials, ep—ethmoid plate, h—hyoid arch, m—mandibular arch, t—trabecular cartilages. |
|
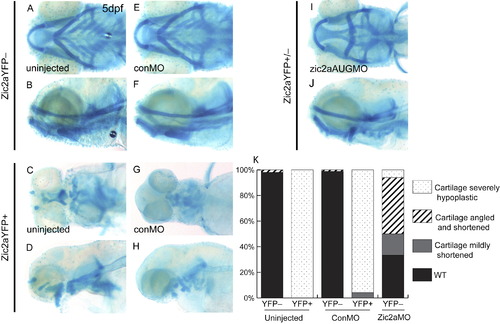
Zic2a translation-blocking morpholino rescues craniofacial defects associated with elevated Zic2a levels. Alcian blue-stained cartilage in heat-shocked Tg(hsp70l:Gal4VP16);Tg(11XUAS:zic2aYFP) embryos at 5 dpf. (A–D) HS-induced expression of Zic2aYFP at 10 hpf results in severely shortened and hypoplastic anterior cartilages in uninjected embryos (67/67 embryos, 1 exp.; shown in C,D) (E–H) Zic2aYFP misexpressing control morphants display severe shortening and hypoplasia comparable to uninjected siblings (23/24, 2 exp.; shown in G,H). (I,J) Zic2aYPF misexpressing embryos injected with Zic2a morpholino were either wild-type (28/135, 2 exp.) or displayed variable craniofacial defects including shortening of anterior cartilages with an incorrectly angled hyoid arch (58/136, 2 exp.; shown in I,J) or more severe hypoplasia (12/136, 2 exp.; not shown). Zic2a overexpressor morphants did not display detectable amounts of the induced Zic2aYFP fusion protein (132/136, 2 exp.). (K) Penetrance of cartilage defects at 5 dpf following zic2aAUGMO injection and Zic2aYFP induction at 10 hpf. A,C,E,G,I are ventral views anterior to the left. B,D,F,H,J are lateral views. |
|
Transplanted cells contribute to neural crest migratory streams and craniofacial cartilage. (A, B) Eight micron confocal Z-stacks of host sox10:GFP+ embryos with alexa568+ transplanted cells. (A) Transplanted control morphant cells colocalize with sox10:GFP-positive cells in migratory streams in 5/7 embryos analyzed. (B) Transplanted zic2a morphant cells colocalize with sox10:GFP-positive cells in migratory streams in 3/5 embryos analyzed. (C–E) 4 dpf embryos stained for alcian blue and biotin. (C) Zic2a morphant embryos have typical bilateral defects in the pharyngeal cartilages. (D) Zic2a morphant embryos that receive transplanted control morphant cells frequently display more severe defects on one side (see arrows, 9/15, 1 exp.). (E) Biotin-positive control morphant cell incorporation in the ethmoid plate of a zic2a morphant host embryo (see arrow, 2/15, 1 exp.). |
|
Zic2b is not required for patterning the Hh target ptch2. Zic2 morphant embryos stained by ISH for ptch2 at 24 hpf. (A,B) Zic2a knockdown does not affect expression of ptch2 (see Sanek and Grinblat, 2008; 16/18, 1 exp.). (C,D) Ptch2 expression is similarly unaffected in zic2b morphants (15/16, 1exp.) and double zic2 morphants (13/15, 1 exp.). A–D are lateral views, anterior to the left. |
|
The oropharynx is mildly mispatterned in zic2a morphants. Embryos stained by ISH for pitx2a or apoeb at 3 dpf. (A,B) Pitx2a expression is increased in the pharyngeal epithelium following Zic2a knockdown (49/56, 4 exp.). (C,D). apoeb, another marker of the oropharyngeal epithlium, shows a similar reduction in stomodeal size in moderately affected zic2a morphants (6/23, 2 exp.), while more severely affected embryos have no apoeb expression in the oropharnx (15/23, not shown). (E,F) apoeb is mildly mispatterned in zic2aAUGMO-injected embryos, either with increased, continual expression in the ventral stomodeum (8/48, 2 exp, arrows in F) or elongation of the stomodeum (25/48, 2 expt., not shown). (G,H) After Zic2aYFP induction at 10 hpf, the stomodeal apoeb expression domain is variably mispatterned: narrowed and lengthened (14/49, 2 exp., see H), tightened into a smaller ring (7/49, 2 exp.) or fused into a single spot (25/49, 2 exp.). A,B are lateral views. C–H are ventral views, anterior to the left. |
Reprinted from Developmental Biology, 380(1), Teslaa, J.J., Keller, A.N., Nyholm, M.K., and Grinblat, Y., Zebrafish Zic2a and Zic2b regulate neural crest and craniofacial development, 73-86, Copyright (2013) with permission from Elsevier. Full text @ Dev. Biol.