- Title
-
Generation and validation of a zebrafish model of EAST (Epilepsy, ataxia, sensorineural deafness and tubulopathy) syndrome
- Authors
- Mahmood, F., Mozere, M., Zdebik, A.A., Stanescu, H.C., Tobin, J., Beales, P.L., Kleta, R., Bockenhauer, D., and Russell, C.
- Source
- Full text @ Dis. Model. Mech.
|
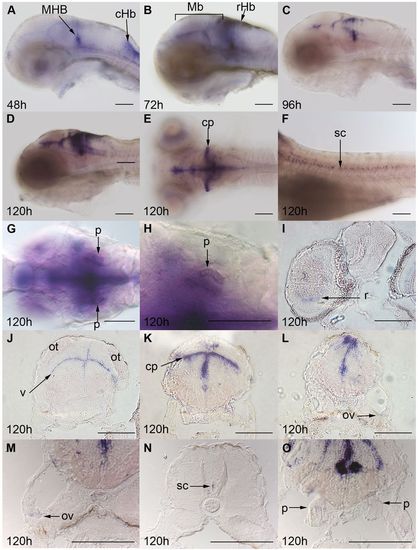
ZF kcnj10a is expressed in brain, otic vesicles and pronephros. At 24 hpf, kcnj10a expression cannot be detected by in situ hybridisation (not shown) but, by 48 hpf (A), kcnj10a is expressed strongly in the mid-hindbrain boundary (MHB) and caudal hindbrain (cHb), and weakly in the midbrain and rostral hindbrain. (A–F) Until 120 hpf, kcnj10a expression becomes stronger, especially in the midbrain and rostral hindbrain (see Mb and rHb labels in B), and in the cerebellum (cp in E). More posteriorly, expression can be seen in the spinal cord (sc in F), but there is no evidence of expression in the lateral line. (I–O) Transverse sections at 120 hpf demonstrate kcnj10a expression in the inner nuclear layer of the retina (r in I) and reveal the majority of midbrain (J), hindbrain (K,L,M) and spinal cord (N) expression to be adjacent to the ventricles (for example, v in J). kcnj10a is also expressed in the cerebellum (cp in E,K), otic vesicle (ov in L,M) and weakly in the pronephros (p in G,H,O). (A–H) Whole-mount embryos with H being a higher magnification image of the pronephros (p) shown in G. (A–D,F) Lateral views with anterior to the left and dorsal up. (E,G,H) Dorsal views with anterior to the left. (I–L) Progressively more posterior transverse sections. (M–O) Higher magnification images of the otic vesicle, spinal cord and pronephros. ot, optic tectum. Scale bars: 100 μm. |
|
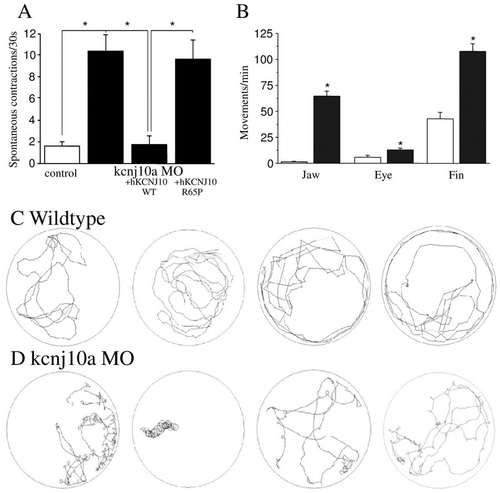
kcnj10a morphant ZF display movement defects. (A) Morphants showed an increased frequency of spontaneous contractions at 30 hpf, and this increase could be rescued by co-injection of normal human KCNJ10 cRNA, but not R65P mutant cRNA (n=10-15 per experimental condition). (B) At 120 hpf, we observed abnormal movements with a significantly higher frequency of jaw, eye and fin movements in kcnj10a morphants (black bars, n=7) than in controls (white bars, n=5). (C,D) Shown are 3-minute tracks from four WT (C) and four kcnj10a morphant (D) ZF at 120 hpf after being startled by touch. kcnj10a morphants (D) exhibit circling locomotion with frequent ′loops′ around their vertical axis. *P<0.05. |
|
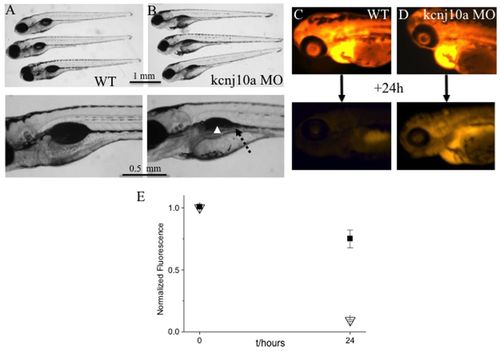
kcnj10a morphants have kidney defects. (A,B) The upper images shows three 120-hpf WT larvae (A) and three kcnj10a morphants (B), some of which have pericardial edema. The lower image is an enlargement of the swim bladder and pronephric duct area. In morphants, the pronephric duct is visible because it is dilated (dotted arrow) and, although partially obscured by pigment, the swim bladder is not visible (arrowhead). (C,D) The upper and lower images show a 72-hpf WT (C) and a kcnj10a morphant (D) immediately after fluorescent dextran injection (upper image) and 24 hours later (lower image). Although baseline fluorescence immediately after injection was comparable in WT and morphant, there was significantly higher remaining fluorescence after 24 hours in morphant (D) compared with WT (C) ZF. (E) Graph of measured fluorescence in WT (triangles; n=9) and morphant ZF (squares; n=12). y-axis shows fluorescence intensity normalised for the baseline measurement in WT. x-axis shows time (hours). PHENOTYPE:
|
|
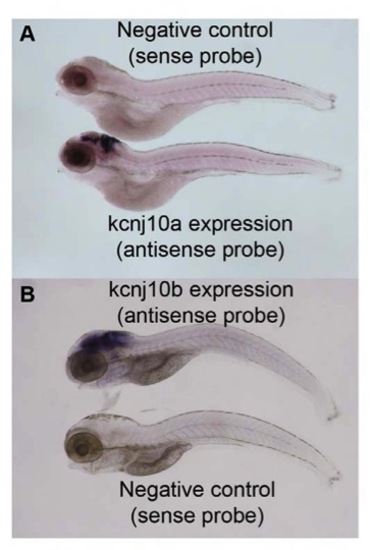
The in situ hybridisation probes are specific. In situ hybridisation on 120 hpf WT ZF shows that the sense probe, which acts as a negative control, does not produce any specific staining. The antisense probe, however, produces specific staining in the central nervous system and indicates the expression of the gene. This is demonstrated both for kcnj10a (A) and kcnj10b (B). Scale bar=100 μm. |
|
Kcnj10b is expressed in the brain and otic vesicles. Expression of kcnj10b, detected by in situ hybridization, is not evident at 24 hpf but becomes weakly ubiquitously expressed throughout the central nervous system by 30 hpf (A), which remains ubiquitous at 48 hpf (B), after which is becomes more highly expressed along ventricles and axon tracts throughout the midbrain and hindbrain, including the cerebellar plate (cp in F), whilst maintaining quite low levels of expression throughout the brain, including the retina (C-G). Expression is evident in the otic vesicle at 120 hpf (H), but is not evident in the pronephros, spinal cord, or lateral line. All images are whole mount with anterior to the left. All images show a lateral view except F, which is a dorsal view. Scale bar=100 μm. |
|
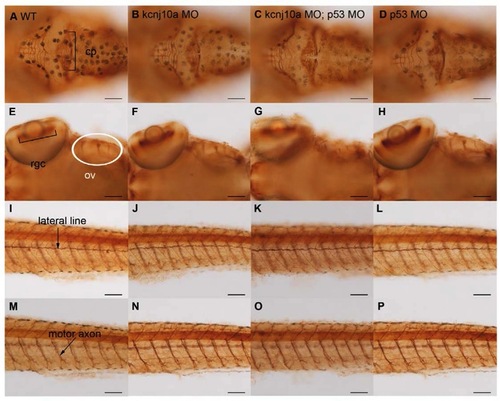
Kcnj10a knockdown does not cause off-target effects or significant anatomical changes to the nervous system. Immunohistochemistry for anti-acetylated α tubulin at 120 hpf reveals axons and dendrites and labels the cerebellar plate (cp in A), retinal ganglion cells (rgc in E), ganglia of otic vesicle (ov in E), lateral line (arrow in I) and motor axons (arrow in M). Comparison of wildtype (A, E, I and M) to kcnj10a morphants (B, F, J and N), kcnj10a and p53 double morphants (C, G, K and O), and p53 morphants (D, H, L P) reveals no significant differences in these structures. Scale bar=100 μm. PHENOTYPE:
|
|
Knockdown of kcnj10b results in specific early morphological defects. (A-D) Live 30 hpf images. Kcnj10b morphants (A) and kcnj10b/p53 double morphants (B) display the same abnormal phenotype, albeit slightly weaker in kcnj10b/p53 double morphants. Scale bar=0.5 mm. PHENOTYPE:
|

Unillustrated author statements |