- Title
-
The Role of egr1 in Early Zebrafish Retinogenesis
- Authors
- Zhang, L., Cho, J., Ptak, D., and Leung, Y.F.
- Source
- Full text @ PLoS One
|
The expression dynamics of egr1 during zebrafish retinogenesis. A time-series whole-mount in situ hybridization was performed to detect the expression pattern of egr1 in the WT retina. The signal of egr1 was first detected in the anterior-ventral retina at 40 hpf (A, arrow). Then, egr1 expression spread to the dorsal retina at 52 hpf (B, arrows). At 72 hpf (C & E), strong signal was detected in the AC and GC regions. Occasionally, positive staining was observed in the HC and PR regions, but it did not become prominent in the peripheral outer retina until 120 hpf (D & F). At this stage, the signal was relatively intense in the GCL and AC region. GC: ganglion cells; AC: amacrine cells; HC: horizontal cells; PR: photoreceptors. Scale bars = 50 µm. |
|
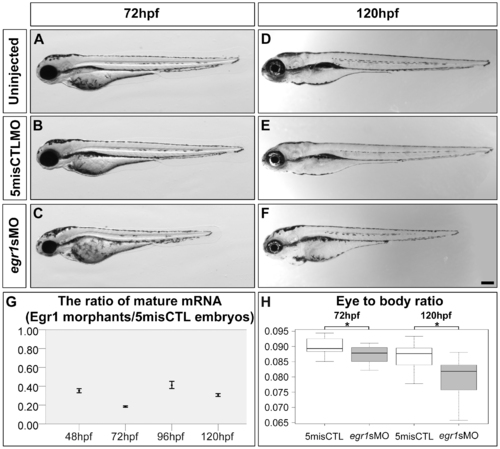
Egr1 was substantially knocked down by antisense morpholino up to 120 hpf. Compared with the uninjected embryos (uninjected; A) and controls (5misCTLMO; B) which had no obvious change in gross morphology, 89.3% (134/150) of the Egr1 morphants (egr1sMO; C) displayed a reduced eye size and a shortened body trunk at 72 hpf. This problem persisted to 120 hpf, at which the Egr1 morphants (F) were still noticeably shorter than the controls (D & E). Scale bar = 200 µm. The amount of the mature egr1 mRNA in the samples was measured by qPCR in order to determine the efficiency of the splice-blocking effect. The ratio and ratio range of the mRNA between Egr1 morphants and controls at 48, 72, 96 and 120 hpf were plotted in (G). The calculation of ratio and ratio range was conducted with the standard ΔΔCt method [47] as described in Methods. Overall, only about 20–40% of mature mRNA was detected in the Egr1 morphants until 5 dpf. (H) A boxplot of the eye-to-body ratios of the controls and Egr1 morphants. The anterior-posterior length of the eyes, when normalized by the body length, revealed a specific reduction of the eye size in the Egr1 morphants at both 72 and 120 hpf. Asterisks: p<0.05. PHENOTYPE:
|
|
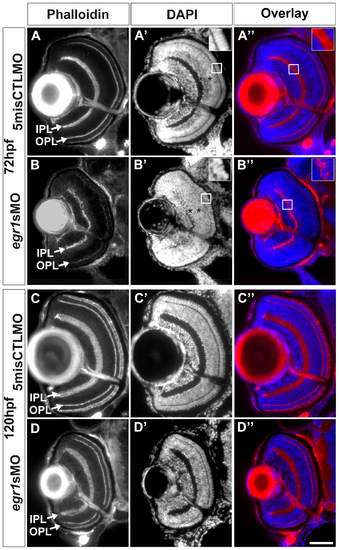
Egr1 knockdown compromised retinal differentiation and lamination at 72 hpf, and these defects were mostly resolved by 120 hpf. The retinal histology of the Egr1-morphant retinas was analyzed by immunofluorescence. (A & B) The IPL and OPL (arrows) that were stained with phalloidin were thinner and irregular in the Egr1 morphants (egr1sMO) compared with the controls (5misCTLMO) at 72 hpf. A similar observation of the plexiform layer formation was also made with the DAPI nuclei stain on the same sections (A′ and B′). The DAPI stain also revealed issues in the differentiation of INL and ONL. For example, while a more intense apical sub-layer and a less intense basal sub-layer were observed in the INL of the controls (A′, asterisks), this distinction was not apparent in the Egr1 morphants (B′, asterisks). This suggests that the differentiation of the INL was compromised in the morphant retinas. Further, the nuclei in the ONL of the Egr1 morphants (B′, inset) were less elongated than that in the controls (A′, inset), suggesting that PR differentiation was affected by Egr1 knockdown. The overlay pictures of phalloidin and DAPI also demonstrate that the irregularity of the IPL was caused by mis-placed cells in the IPL in the Egr1 morphants (B′′, inset); while no mis-placed cells were found in the controls (A′′, inset). By 120 hpf, many of these differentiation problems in the Egr1-morphant retinas were largely resolved. For example, the IPL and OPL were more regular and their phalloidin staining was more intense (D) and was comparable to the controls (C). Nonetheless, the IPL was still thinner in the Egr1 morphants. In addition, the PRs in the Egr1 morphants was not stained as intensely by phalloidin as the controls, even though the differentiation of PRs became relatively normal at this stage (see Figure 7). For all sections, the lens is on the left and dorsal is up. IPL: inner plexiform layer; OPL: outer plexiform layer. Scale bar = 50 µm. PHENOTYPE:
|
|
Immunohistochemical analysis of the INL cells in the Egr1-morphant retinas. Immunohistochemical analysis of the INL cells in the controls (5misCTLMO) and Egr1 morphants (egr1sMO) was performed with several cell markers at 72 and 120 hpf. These include anti-5E11 (5E11; A-D), anti-parvalbumin (Parv; E-H), anti-GABA (GABA; I-L) and anti-Islet1 (Islet1; M-P) for ACs; anti-PKC²1 (PKC; Q-T) for BCs; anti-GS (GS; U-X) for MCs; and Islet1 and anti-Prox1 (Prox1; Y-AB) for HCs. In short, the analysis has revealed that Egr1 knockdown specifically compromised the differentiation of Parv+ and GABA+ ACs. See text, Table 1 and 2 for further discussion and additional results for the specific effects on HCs differentiation in Figure 6. For all sections, the lens is on the left and dorsal is up. Scale bar = 50 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Immunohistochemical analysis of the GCs in the Egr1-morphant retinas. Immunohistochemical analysis of the GCs in the controls (5misCTLMO) and Egr1 morphants (egr1sMO) was performed by anti-zn8 (zn8; green) at 72 hpf (A & B) and 120 hpf (C & D). Phalloidin (red) was used as a counterstain to highlight the plexiform layers. A whole-eye section is shown at the top for each condition, while the magnified view of a selected region (white box) on the dorsal side of the optic nerve is shown at the bottom. The analysis has indicated that Egr1 knockdown suppressed the early dendritic outgrowth of GCs into the IPL at 72hpf (B), which was irregular at this stage. In addition, the cell number per retinal area was not different between the two groups. This defect was largely resolved by 120 hpf, despite the IPL was still thinner as shown in Figure 3. This suggests that there were still defects in differentiation of cells that projected neurites into the IPL. One possible cause of the defect is the differentiation problem of ACs as shown in Figure 4. See text, Table 1 and 2 for further discussion. For the whole-eye sections, the lens is on the left and dorsal is up. Scale bar = 50 µm for the whole-eye sections and 25 µm for the selected regions. EXPRESSION / LABELING:
PHENOTYPE:
|
|
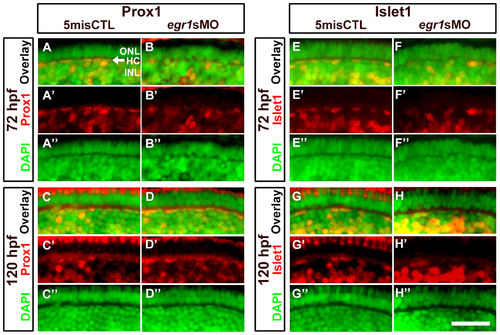
Immunohistochemical analysis of the HCs in the Egr1-morphant retinas. A magnified view of the immunostaining results of HCs in the controls (5misCTLMO) and Egr1 morphants (egr1sMO) with Prox1 (A-D) and Islet1 (E-H) at 72 and 120 hpf. These selected regions correspond to the white boxes as shown in Figure 4. Prox1+ and Islet1+ cells are shown in red, while the DAPI nuclei counterstain is shown in green. The location of HCs is indicated by an arrow in A. See text, Table 1 and 2 for further discussion. In all pictures, the apical retina is to the top and dorsal is to the left. HC: horizontal cells; INL: inner nuclear layer; ONL: outer nuclear layer. Scale bar = 50 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
PR differentiation was delayed in the Egr1-morphant retinas. Immunohistochemical analysis of the PRs in the controls (5misCTLMO) and Egr1 morphants (egr1sMO) was performed with zpr1 (red-green double cones) and zpr3 (rods) at 72 hpf (A-D) and 120 (E-H) hpf. The signal of zpr1+ and zpr3+ cells was detected in the whole ONL of the controls at 72 hpf (A & C), while they were substantially reduced and restricted to a small region on the ventral ONL in the Egr1 morphants (B & D). Four staining types were defined as follows: Type 1: d ¼, 2: d ½, 3: d ¾, 4 = full retina. In these example images, the controls are staining Type 4 while the morphant images are staining Type 1. By 120 hpf, the differentiation of the zpr1+ and zpr3+ cells in the Egr1-morphant retinas (F & H) became more comparable to the controls (E & G). For all sections, the lens is on the left and dorsal is up. Scale bar = 50 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
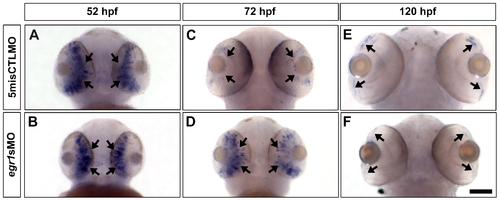
The expression of ptf1a, a TF that specifies ACs and HCs, was abnormal in the Egr1-morphant retinas. Whole-mount in situ hybridization of ptf1a was performed with the controls (5misCTLMO) and Egr1 morphants (egr1sMO) collected at 52, 72 and 120 hpf. At 52 hpf, ptf1a was primarily expressed in the differentiating retinal neuroepithelium (A & B, arrows) in both types of samples. By 72 hpf, the expression of ptf1a was restricted to the proliferative MZ in the controls (C, arrows), while its expression was maintained in the developing central retina in the Egr1 morphants (D, arrows). This ectopic expression was transient, as ptf1a was finally expressed in MZ in the Egr1 morphants (F, arrows) in a very comparable manner as the controls (E, arrows). The ventral view of the embryos is shown in all pictures. Scale bar = 100 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Amacrine cells immunolabeled by Parv and GABA markers. (Top) An overlay image of GABA+ (green) and Parv+ (red) cells in a normal WT retina at 72 hpf. (Bottom) A magnified view of the white box at the top. From left to right: GABA, Parv and the overlay image. Many of the Parv+ AC cell bodies were also GABA+ (white arrows), suggesting they might be a subset of GABAergic ACs. Note that there were overlapping and non-overlapping GABA+ and Parv+ regions in the IPL, suggesting that these ACs projected to different sub-laminae in the IPL. Scale bar = 50 µm for the top image and 10 µm for the bottom images. |
|
In situ hybridization of opsinsat 72 hpf. In situ hybridization of opn1lw1 (red; A & B), opn1sw2 (blue; C & D), opn1sw1 (uv; E & F) and rhodopsin (rho; G & H) was conducted with the controls (5misCTLMO) and Egr1 morphants (egr1sMO) collected at 72 hpf. The staining of four opsins were strongly detected in the whole ONL of the control retinas (A, C, E and G), while their signal in the Egr1 morphants was restricted to the ventral patch and/or a few ONL cells (arrows in B, D, F and H). The ventral view of the embryos is shown in all pictures. To quantify the signal intensity of in situ hybridization, the number of embryos with a specific level of staining (Type 1 - ventral patch staining only, Type 2 – ventral patch staining plus some central PR layer staining, and Type 3 – ventral patch plus full PR layer staining) was counted and analyzed by Mann-Whitney test. The results show that there was a difference in the staining type between the controls and Egr1 morphants for all four opsins ([red opsin]: control counts (type 1–3): 0, 0, 12; Egr1-morphant counts: 5, 13, 0; U = 0, p-value < 0.001; [blue opsin]: control counts: 0, 0, 12; Egr1-morphant counts: 11, 7, 0; U = 0, p-value < 0.001; [uv opsin]: control counts: 0, 0, 12; Egr1-morphant counts: 13, 5, 1; U = 6, p-value < 0.001; [rho]: control counts: 0, 0, 9; Egr1-morphant counts: 15, 5, 0; U = 0, p-value < 0.001). In this figure, all controls are staining Type 3 while all Egr1 morphants are staining Type 2. Note that the effect of Egr1 knockdown on PR differentiation is likely caused by a delay in development, as the immunostaining of PR markers at 120 hpf shows that the differentiation of PRs in the Egr1 morphants was comparable to the controls (Figure 7). Scale bar = 100 µm. |
|
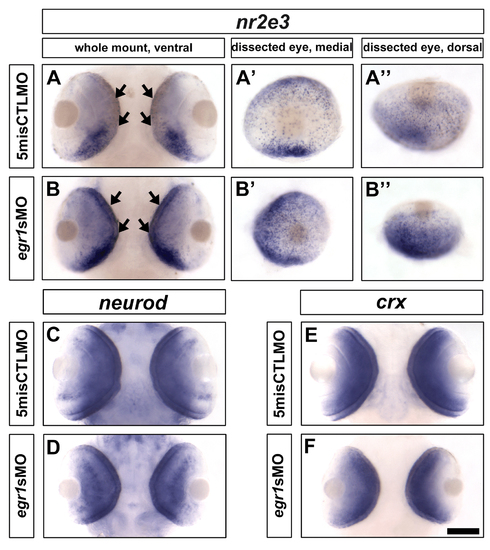
In situ hybridization of nr2e3, neurod and crx at 72 hpf. (A & B) The staining of nr2e3 in the Egr1-morphant retinas was higher from the ventral (B) and dorsal (B′′) views compared with the controls (A & A′′). From the medial view, the PRs that were stained as individual dots were widely distributed in the Egr1-morphant retinas (B′), while they were relatively sparse in the control retinas, especially in the central region (A′). For neurod and crx, their expression patterns and levels were comparable between the control (C & E) and Egr1-morphant (E & F) retinas. Thus, these observations suggest that egr1 negatively regulates nr2e3 but not neurod and crx at 72 hpf. Nonetheless, since PRs ultimately differentiated relatively normally in the Egr1 morphants at 120 hpf (Figure 7), the results are more consistent with the possibility that the development of PRs was delayed in the morphants. Scale bar = 100 µm. |