- Title
-
Fibronectin mediates correct positioning of the interrenal organ in zebrafish
- Authors
- Chou, C.W., Chiu, C.H., and Liu, Y.W.
- Source
- Full text @ Dev. Dyn.
|
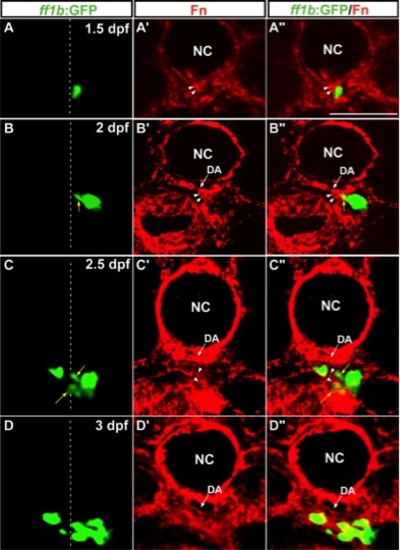
The distribution of Fn around migrating interrenal cells. Interrenal cells (GFP as driven by ff1b promoter in A–D and A′′–D′′) formed a protruding extension at 2 dpf (B, B′′) and dispersed clusters at 2.5 (C, C′′) and 3 dpf (D, D′′), on transverse sections of Tg(ff1bEx2:GFP) embryos. Migrating interrenal cell clusters (denoted by yellow arrows) were closely associated with the Fn protein (red fluorescence in A′–D′ and A′′–D′′; Fn enrichment in the interrenal microenvironment highlighted by white arrowheads) throughout the medial extension process of the interrenal tissue. All sections are oriented with the dorsal side toward the top. White dotted lines indicate the position of the midline. NC, notochord; DA, dorsal aorta. Scale bar = 50 μM. EXPRESSION / LABELING:
|
|
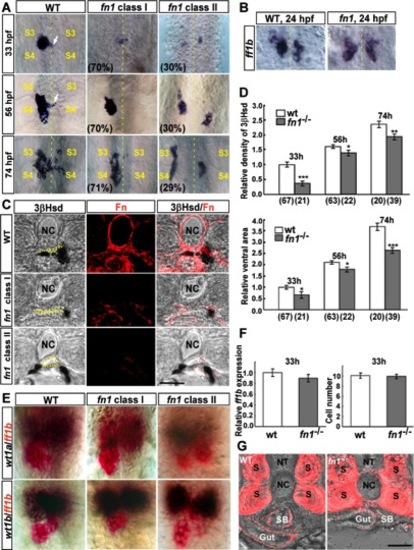
The analysis of interrenal morphogenesis in the fn1 mutant. A: Morphogenetic movements of the steroidogenic interrenal tissue in the fn1 mutant (fn1) and its wild type siblings (WT) at 33, 56, and 74 hpf were detected by whole-mount 3β-Hsd staining. Ventral flat-mount views of deyolked samples showed that the steroidogenic interrenal tissue appeared as a single aggregated cell cluster in the wild-type embryo, with the medially-migrating cells forming a protruding extension (white arrows) evident as early as 33 hpf, at the level between the third (S3) and fourth (S4) somites, which was extended across the midline (yellow dotted lines) by 56 hpf and became more loosely associated at 74 hpf. The medial extension and dispersion of single interrenal cell cluster was defective in the class I fn1 mutant, while the class II mutant displayed a more severe phenotype where bilateral interrenal primordia did not converge. B: Bilateral interrenal clusters expressing ff1b transcripts were detected in both the fn1 mutant and its wild-type sibling at the stage of 24 hpf, as assayed by an ISH analysis. C: The interrenal migration phenotype of the fn1 mutant at 72 hpf, which displayed disrupted Fn expression (red), was further demonstrated by vibratome sections with the dorsal side oriented toward the top. The lumen of the DA is marked by yellow dots. D: Relative density (top chart) and area (bottom chart) of steroidogenic interrenal tissues as assessed by 3βHsd activity staining from the ventral surface, in the fn1 mutant compared with its wild-type sibling control. The number of embryos for each population is indicated in parentheses. Both density and area of 3βHsd activity-positive interrenal cells were mildly yet significantly reduced in the fn1 mutant. *P < 0.05; **P < 0.005; ***P < 10−5. E: Double ISH assays showing the colocalization of ff1b (red) with wt1a and wt1b (dark brown), respectively, at 33 hpf in the fn1 mutant as well as its wild-type sibling. F: The Fast Red fluorescent intensity of ff1b ISH signals, and the number of ff1b-expressing interrenal cells as measured from the ventral surface, are quantified in left and right charts, respectively. Expression levels of ff1b transcripts, as well as the number of ff1b-expressing interrenal cells, are essentially invariable between the fn1 mutant (n = 25) and its wildtype siblings (n = 18) at the early stage of interrenal differentiation. G: The morphology of the developing gut tube in the fn1 mutant. Vibratome sections at the level of anterior gut region in the fn1 mutant (fn1-/-) as well as its wild-type sibling (WT) were stained with the antibody against Acta2 for demarcating smooth muscle cells located at the outer layers of gut tube (Gut) and swim bladder (SB), while the antibody also detected myofibrils at the somites (S). Morphology but not laterality of the gut tube is affected in the absence of Fn. NT, neural tube. NC, notochord. Scale bar = 50 μM. EXPRESSION / LABELING:
PHENOTYPE:
|
|
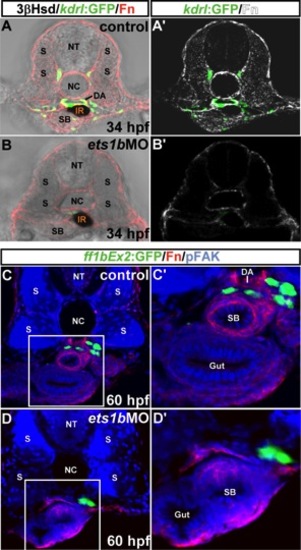
The effect of ets1bMO on the Fn distribution in the interrenal region. Top: Transverse sections of Tg(kdrl:GFP)s843 embryos either uninjected (A, A′) or injected with ets1bMO (B, B′) were harvested at 34 hpf and assayed for 3β-Hsd activity (black), GFP (green), and Fn expression (red in A, B; white in A′, B′). The result of ets1b morphant in B, B′ is a representative of 9 sectioned samples, which all showed disrupted vasculature at the level of the midtrunk. Bottom: Transverse sections of Tg(ff1bEx2:GFP) embryos either uninjected (C, C′) or injected with ets1bMO (D, D′) were harvested at 60 hpf and assayed for GFP (green), Fn expression (red), and pFAK (blue). C′, D′ are magnified views of the outlined areas in C, D. The result of ets1b morphant in D, D′ is a representative of 20 sectioned samples. The accumulation of Fn in the interrenal microenvironment is severely reduced in the ets1b morphant. All sections are oriented with the dorsal side toward the top. DA, dorsal aorta; IR, interrenal tissue; NC, notochord; NT, neural tube; S, somite; SB, swim bladder. |
|
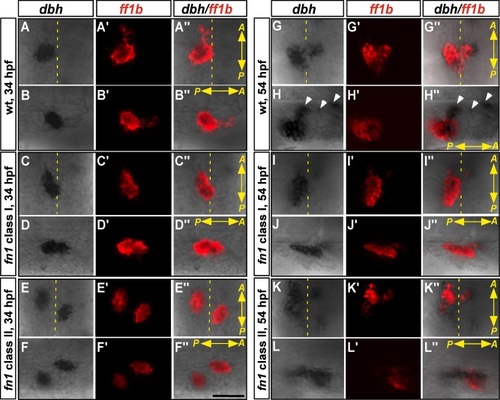
The interaction between interrenal and chromaffin cells in the fn1 mutant at 34 and 54 hpf. Double ISH assays showing the colocalization of ff1b (red) with dbh (black) in the class I and II fn1 mutants, as well as their wild-type siblings. Ventral flat mount (A–A′′, C–C′′, E–E′′, G–G′′, I–I′′, K–K′′) and lateral (B–B′′, D–D′′, F–F′′, H–H′′, J–J′′, L–L″) views are shown for the representative embryo of each phenotypic type (n =17, 10, and 24 for the 34-hpf fn1 mutant class I, class II, and their wild-type siblings, respectively; n = 6, 3, and 18 for the 54-hpf fn1 mutant class I, class II, and their wild-type siblings, respectively). The anterior (A) versus posterior (P) orientation of each sample is indicated, and the midline is marked by yellow dotted lines. The embryos in the lateral view panels are oriented with the dorsal side to the top, and migrating chromaffin cell clusters are highlighted by white arrowheads. The initial interaction between interrenal and chromaffin cell populations appears normal; while the continuous recruitment of chromaffin cells to the interrenal region is defective in the fn1 mutant. Scale bar = 50 μM. |
|
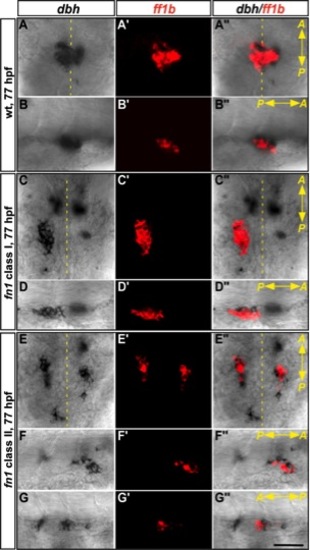
The interaction between interrenal and chromaffin cells in the fn1 mutant at 77 hpf. Double ISH assays showing the colocalization of ff1b (red) with dbh (black) at 77 hpf in the class I and II fn1 mutants, as well as their wild-type sibling. Ventral flat mount (A–A′′, C–C′′, E–E′′) and lateral (B–B′′, D–D′′, F–F′′, G–G″) views are shown for the representative embryo of each phenotypic type (n = 14, 7, and 58 for the fn1 mutant class I, class II, and their wild-type siblings respectively). The anterior (A) versus posterior (P) orientation of each sample is indicated. The embryos in the lateral view panels are oriented with the dorsal side to the top. Defective migration and convergence of chromaffin cells in the fn1 mutant result in incomplete interrenal organ assembly. Scale bar = 50 μM. EXPRESSION / LABELING:
PHENOTYPE:
|
|
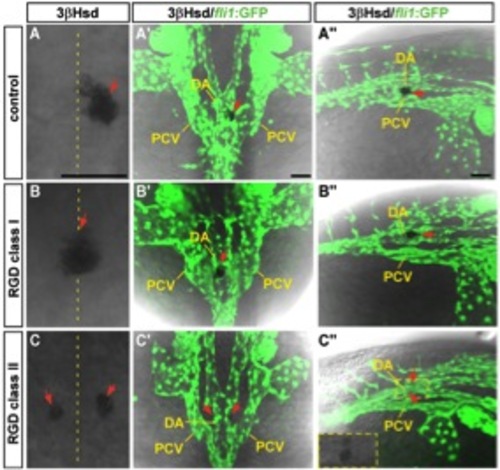
The effect of RGD peptide treatment on the interrenal tissue and the peri-interrenal vasculature. Sets of confocal images display the interrenal tissue as detected by 3β-Hsd activity staining (red arrows), and the midtrunk vasculature by fli1 promoter-driven green fluorescence, of 32-hpf Tg(fli1:EGFP)y1 embryos as treated with RGD peptide from 16 to 32 hpf. A–C, A′′–C′′: Dorsal views showing the midtrunk of the representative embryo for each phenotypic class, with anterior oriented to the top. A′′–C′′: Dorso-lateral views of the same embryo with anterior to the right. The interrenal region in C′′ is outlined, with its enlarged bright field image shown in the inset. The interrenal tissue in RGD-treated embryos displays affected laterality in the class I phenotype, and disrupted midline fusion in the class II phenotype. DA, dorsal aorta. PCV, posterior cardinal vein. Scale bar = 50 μM. |