- Title
-
Loss of ascl1a prevents secretory cell differentiation within the zebrafish intestinal epithelium resulting in a loss of distal intestinal motility
- Authors
- Roach, G., Heath Wallace, R., Cameron, A., Emrah Ozel, R., Hongay, C.F., Baral, R., Andreescu, S., and Wallace, K.N.
- Source
- Full text @ Dev. Biol.
|
Intestinal expression of ascl1a. ascl1a is expressed in a few intestinal epithelial cells at 44 hpf throughout the length of the intestine ((A), arrow points to intestine). Higher magnification (B) shows individual epithelial cells expressing ascl1a, which is sparse and light at this stage. The number of cells expressing ascl1a increases by 48 hpf ((C), arrow points to intestine) and higher magnification (D). This changes at 50 hpf (E), when ascl1a is expressed more evenly throughout the intestinal epithelium, often in clusters of cells (higher magnification in (F)). In cross section at 50 hpf a single section will often display multiple epithelial cells expressing ascl1a (arrows in (I)). Expression of ascl1a is still strong in the intestinal epithelium at 74 hpf ((G), arrow points to intestine) but cells are often less clustered. Cross sections more frequently display single epithelial cells expressing ascl1a (arrow in (J)). All whole mount images ((A)–(H)) are oriented anterior to left and posterior to right. Scale bars: (A), (C), (E), and (G) 200 μm; (B), (D), (F), and (H) 50 μm; (I) and (J) 20 μm. EXPRESSION / LABELING:
|
|
ascl1a-/- 5 dpf embryos lack intestinal epithelial secretory cells. At 5 dpf WT embryos have a narrow diameter throughout the intestinal lumen (A). ascl1a-/- embryos (B) develop an increased luminal diameter in the proximal intestine (arrow), fail to completely utilize the yolk, and fail to inflate the swim bladder while the distal intestine appears normal. The 2F11 intestinal epithelial pan-secretory cell marker reveals numerous secretory cells distributed throughout the length of the 5 dpf intestine (C). Within ascl1a-/- 5 dpf embryos, secretory cells are absent throughout the entire intestine (D). In this preparation residual pancreatic cells are observed on the lateral side of the proximal intestine (arrow in (D) and region enlarged in (D′)). Markers for individual subtypes of secretory cells are also absent in the intestinal epithelium (only the distal intestine is shown in panels (E)–(H)). Goblet cells are distributed only throughout the distal WT intestine (E) as demonstrated by the fluorescently labeled lectin, wheat germ agglutinin but are absent from ascl1a-/- embryos (F). Serotonin (5HT) containing enterochromaffin cells are distributed only throughout the distal intestinal epithelium along with 5HT enteric neurons as demonstrated by anti-5HT immunohistochemistry (G). In ascl1a-/- embryos, enterochromaffin cells are absent; however, there are a few remaining 5HT enteric neurons (arrowheads (H)). Scale bar represents 100 μm. |
|
Intestinal enterocytes differentiate with characteristic markers in ascl1a-/- 5 dpf embryos. Enterocytes differentiate within the ascl1a-/- intestinal epithelium as demonstrated by apical alkaline phosphatase (blue in (B) compared to WT in (A)), apical sodium phosphate transporter (NaPi) (green in (D) compared to WT in (C)), and the tight junction marker ZO-1 (green in (F) compared to WT in (E)). Apical alkaline phosphatase in ascl1a-/- is typically less than WT but NaPi and ZO-1 are present at the same intensity. Scale bar represents 50 μm except in (A) and (B) where the bar is 20 μm. |
|
Intestinal expression of deltaD in WT and ascl1a-/- embryos. deltaD expression in the intestinal epithelium begins on the second day of embryogenesis by 50 hpf ((A), arrow: intestinal expression; arrowhead: neural tube expression out of focal plane) with stronger expression in the proximal intestine. Magnified view of 50 hpf deltaD expression (B) demonstrates small clusters and some isolated intestinal epithelial cells. deltaD expression becomes stronger and more even throughout the intestinal epithelium at 74 hpf ((C): arrow; arrowhead: neural tube expression out of focal plane). Magnified view of 74 hpf (D) demonstrates that deltaD is now expressed in larger clusters and higher numbers of intestinal epithelial cells. ascl1a-/- embryos lack expression of deltaD within the intestinal epithelium at 74 hpf (arrow in (E)) but retain neural tube expression (arrowhead in (E) out of focal plane). Magnified view of ascl1a-/- (F) shows absence of deltaD expression in intestinal epithelium. Within the same ascl1a-/- embryos, deltaD expression in the brain is altered but remains strong (compare WT in (G) to ascl1a-/- in (H)). Anterior is to the left and posterior to the right in all panels. Scale bars: (A), (C), (E), (G), and (H) 200 μm; (B), (D), and (F) 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Intestinal expression of DeltaD in WT and ascl1a-/- embryos. (A) Both mutant and WT 5 dpf intestines and brains were dissected for western blot. Brains for both WT and ascl1a-/- demonstrate similar levels of DeltaD and the loading control β-actin. ascl1a-/- intestines lack DeltaD while there are similar levels of β-actin compared to WT. DeltaD is approximately 100 kD while β-actin is 55 kD. (B) Total protein as visualized by induced fluorescence on stain-free gel. Proteins are visualized after electrophoresis but before transfer to membrane as an alternative loading control to β-actin. The protein gel for DeltaD was run without β-mecaptoethanol (BME) and shown to the left while protein gel for β-actin was run with β-mecaptoethanol (BME) and is shown to the right. The same volume of protein from the same sample was loaded on both the BME and no BME gels. |
|
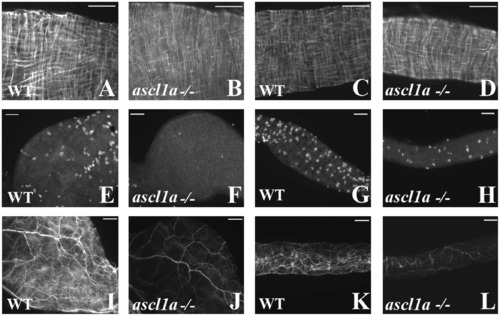
Smooth muscle and enteric neurons in ascl1a-/- at 4–5 dpf. Smooth muscle within the digestive system appears normal from proximal ((B) compared to (A)) to distal ((D) compared to (C)) in ascl1a−/− 5 dpf embryos as determined by desmin immunohistochemistry. Neuronal cell bodies highlighted by Elav 1 are numerous in both the proximal (E) and distal (G) WT intestine at 5 dpf but have been severely reduced in the proximal (F) and distal (H) ascl1a-/- intestine. Neurite density is also severely reduced in both the proximal (J) and distal (L) ascl1a-/- intestine when compared to WT proximal (I) and distal (K) as shown with acetylated tubulin. Scale bars represent 50 μm. |
|
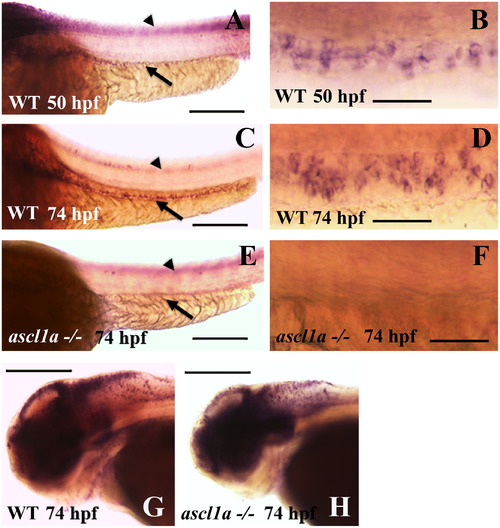
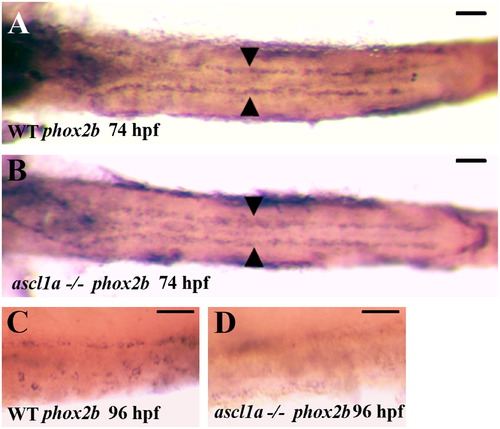
Migration of enteric neural precursors within ascl1a-/- embryos. At 74 hpf enteric neural precursors, as demonstrated by phox2b RNA in situ hybridization, have migrated in two lateral lines to the distal end of the digestive system (A). In genotyped ascl1a-/- embryos, enteric neural precursors also migrate in similar duplicate lateral lines to reach the distal intestine at 74 hpf (B) similar to WT (A). At 96 hpf, phox2b is distributed around the circumference of the WT intestine (C) while they are no longer present in the ascl1a-/- intestine at the same time (D). Scale bars represent 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Anterograde intestinal motility in ascl1a-/- 5 dpf embryos. Spatiotemporal maps (STMaps) were generated by drawing a box around the area of interest (red box in (A), (C), and (E)). Just distal to the swim bladder there is a region that generates both retrograde and anterograde contractions. Length and start position of retrograde and anterograde contractions are indicated by arrows (R-retrograde and A-anterograde). (B) WT STMap generated from area of interest in panel (A). Individual contractions are identified by changes in opacity, which create darker bands and travel downwards over time (indicated at side of STMap). Velocity is calculated using the slope of the line representing the contraction (indicated by blue line), and distance is calculated by measuring the length of the line (indicated by yellow line). Motility is recorded for ten minutes. The origin of retrograde and anterograde contractions are indicated at the top of the panel. Retrograde contractions are more frequent than anterograde contractions. ascl1a -/- embryos (C) with a similar region of interest (red box) generate an STMap (D) with only retrograde contractions and an absence of anterograde contractions. Upon addition of serotonin to ascl1a-/- embryos (E), anterograde contractions (F) are restored at a similar frequency, distance, and velocity to WT (compare anterograde contractions in (F)–(B)). PHENOTYPE:
|
Reprinted from Developmental Biology, 376(2), Roach, G., Heath Wallace, R., Cameron, A., Emrah Ozel, R., Hongay, C.F., Baral, R., Andreescu, S., and Wallace, K.N., Loss of ascl1a prevents secretory cell differentiation within the zebrafish intestinal epithelium resulting in a loss of distal intestinal motility, 171-186, Copyright (2013) with permission from Elsevier. Full text @ Dev. Biol.