- Title
-
Axon tracts guide zebrafish facial branchiomotor neuron migration through the hindbrain
- Authors
- Wanner, S.J., and Prince, V.E.
- Source
- Full text @ Development
|
FBMNs migrate in close apposition to the MLF. (A-C) Maximum projection dorsal views of Tg(zCREST1:membRFP) embryos immunostained using zn-12 antibody show that FBMNs lie in close proximity to the MLF throughout their migration. zn-12 (green) labels the MLF (white arrowheads) and lateral longitudinal fasciculus (LLF, yellow arrowheads). (A) At 20 hpf, FBMNs (red) have migrated into r5 and the first FBMNs to migrate have reached r6 (arrows). (B) By 22 hpf, migration has continued with more FBMNs reaching r6. (C) At 24 hpf, FBMN migration into r6 continues. (D-F) Transverse sections of zCREST1:membRFP embryos at r5 stained using zn-12 show direct interaction between the MLF and FBMNs (white arrowheads) throughout FBMN migration at (D) 20 hpf, (E) 22 hpf and (F) 24 hpf. Asterisks highlight the otic vesicle. Broken lines indicate r5 boundaries for all figures. Scale bar: 20 μm. EXPRESSION / LABELING:
|
|
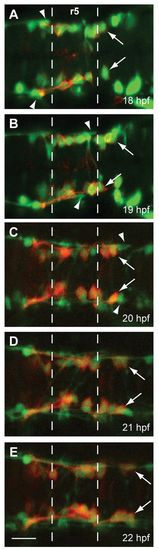
FBMN migration into r5 and r6 precedes the MLF entering the r4-r6 hindbrain region. Time-lapse imaging of Tg(zCREST1:membRFP/HuC-GFP) embryos from 18-22 hpf. (A-E) Still images taken at 1-hour intervals from two representative time-lapse movies: (A,B) a 18-20 hpf movie; (C-E) a 20-22 hpf movie. Arrows indicate the first FBMN to migrate. Arrowheads indicate the growth cone of the first MLF axon to enter the hindbrain. Scale bar: 20 μm. EXPRESSION / LABELING:
|
|
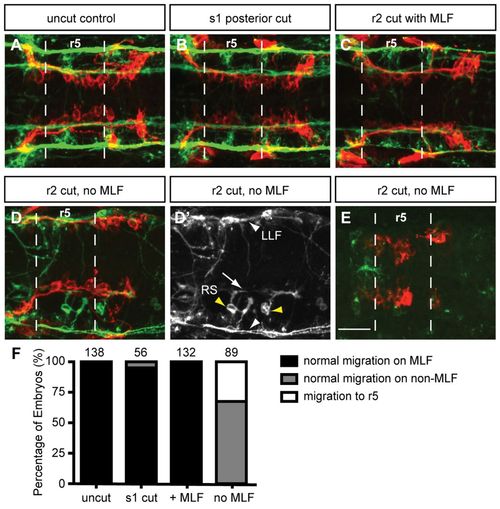
Loss of MLF axons in the hindbrain blocks FBMN migration. Maximum projection dorsal views of FBMNs (red) and the MLF (green) in 22 hpf Tg(zCREST1:membRFP) embryos stained using zn-12 antibody. (A) Uncut control embryos at 22 hpf show that FBMNs migrate in close proximity to the MLF. (B) Cuts made posterior to the hindbrain at the first somite (s1) do not affect FBMN migration. (C) Incomplete severing at r2 allows normal MLF axon and FBMN migration. (D) Severing of the embryo at r2 often results in FBMN migration into r6 on the LLF or projections of the reticulospinal (RS) neurons. (D′) zn-12 staining labels the LLF (white arrowheads), and the RS neurons (yellow arrowheads) and their projections (arrow). (E) Severing the MLF causes FBMN migration to stall in r5. (F) Percentage of embryos affected by manipulations. The number of embryos scored is indicated for each manipulation. Scale bar: 20 μm. EXPRESSION / LABELING:
|
|
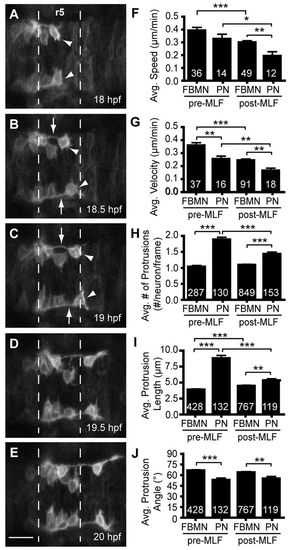
The pioneer neuron behaves differently than follower FBMNs. (A-E) Still images from time-lapse imaging of Tg(zCREST1:membRFP) embryos from 18-22 hpf shown at 30-minute intervals. Arrowheads indicate the pioneer neuron and arrows indicate the trailing axon of the pioneer neuron. (F-J) Analysis of pioneer and follower FBMN behavior before (pre-MLF) and after (post-MLF) the MLF has entered the hindbrain region. (F) Average speed of migration. (G) Average posterior velocity. (H) Average number of protrusions per neuron per 5-minute time point. (I) Average length of protrusions per 5-minute time point. (J) Average angle of protrusions, where 0° is towards the posterior in the direction of migration and 90° is towards the midline. Error bars indicate s.e.m. Significance values: *P<0.05, **P<0.01, ***P<0.001. Scale bar: 20 μm. EXPRESSION / LABELING:
|
|
Pioneer neuron ablation blocks migration of follower FBMNs. (A-C) Maximum projection images of Tg(islet1:GFP) (green) embryos at 24 hpf, after ablation of the pioneer neuron on one side of the embryo at 18-19 hpf. (A) Pioneer neuron ablation blocks follower FBMN migration. (B) Strong partial migration phenotype results in two to four FBMNs reaching r6. (C) Weak partial migration phenotype results in migration of five to eight FBMNs into r6. (D) Ablation of the second FBMN to migrate does not affect FBMN migration in most embryos. (E) Tg(zCREST1:membRFP/pGFP5.3) embryos label FBMNs (red) and neuroepithelial cells in r5 (green), respectively. Ablation of a single r5 neuroepithelial cell in the path of FBMN migration does not affect migration. (F) Ablation of the trailing axon of the pioneer neuron, using Tg(zCREST1:membRFP) embryos, blocks FBMN migration; arrowhead indicates presumed pioneer neuron. (G) Percentage of embryos affected by ablation. Scale bar: 20 μm. EXPRESSION / LABELING:
|
|
Pioneer neuron migration is independent of Cdh2-mediated cell adhesion. (A-E) Still images from a representative time-lapse movie of Tg(zCREST1:membRFP/HuC-GFP) embryos injected with Cdh2MO from 18-22 hpf, shown at 1-hour intervals. Arrows indicate the pioneer neurons. (F-J) Analysis of pioneer and follower FBMN behavior before and after the MLF has entered the hindbrain region in uninjected controls. (K-O) Analysis of Cdh2MO time-lapse data comparing pioneer neuron behavior with follower FBMNs before and after the MLF is present. (F,K) Average speed. (G,L) Average posterior velocity. (H,M) Average number of protrusions per neuron per 5-minute time point. (I,N) Average protrusion length per 5-minute time point. (J,O) Average protrusion angle. Error bars indicate s.e.m. *P<0.05, **P<0.01, ***P<0.001. Scale bar: 20 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Cdh2 is required for maintaining the interaction between the pioneer trailing axon and follower FMBNs. Maximum projection images of Tg(zCREST1:membRFP) embryos. (A-D) In uninjected control embryos at (A) 18 hpf, (B) 20 hpf, (C) 22 hpf and (D) 24 hpf, the trailing axon (arrows) of the pioneer neuron (arrowheads) maintains contact with follower FBMNs. (E-H) In Cdh2MO-injected embryos, the interaction between the trailing axon of the pioneer neuron and follower FBMNs is reduced or absent; examples at (E) 18 hpf, (F) 20 hpf, (G) 22 hpf and (H) 24 hpf. Arrowheads indicate the pioneer neuron. Arrow indicates the trailing axon. Scale bar: 20 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Cdh2 is required for maintaining the interaction between the pioneer trailing axon and follower FMBNs. Maximum projection images of Tg(zCREST1:membRFP) embryos. (A-D) In uninjected control embryos at (A) 18 hpf, (B) 20 hpf, (C) 22 hpf and (D) 24 hpf, the trailing axon (arrows) of the pioneer neuron (arrowheads) maintains contact with follower FBMNs. (E-H) In Cdh2MO-injected embryos, the interaction between the trailing axon of the pioneer neuron and follower FBMNs is reduced or absent; examples at (E) 18 hpf, (F) 20 hpf, (G) 22 hpf and (H) 24 hpf. Arrowheads indicate the pioneer neuron. Arrow indicates the trailing axon. Scale bar: 20 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Early FBMN migration into r5 and r6 precedes both Mauthner and reticulospinal neuron axon projection into the hindbrain. Maximum projection dorsal views of Tg(zCREST:membRFP) embryos immunostained with an acetylated tubulin antibody (A,C,E) and merged images (green; B,D,F). (A,B) At 18 hpf, Mauthner (M) neuron cell bodies are present in r4 (white arrowhead) but their axons are not yet present. Cilia in the lumen of the hindbrain (bracket) as well as stereocilia in the otic vesicle (not shown) are present at this stage, verifying antibody staining efficacy. (C,D) At 20 hpf, axons of the Mauthner neurons have begun to project towards the hindbrain midline (white arrow). Additionally, reticulospinal (RS) neuron cell bodies are present (yellow arrowhead) and have begun to project their axons toward the MLF. (E,F) At 22 hpf the axons of the Mauthner neurons have crossed at the midline in r5 (white arrow) and will soon contribute to the MLF axon tract (white arrowhead). RS neurons (yellow arrowhead) have sent out projections that have contributed to the MLF (yellow arrow). |
|
Blocking the MLF does not affect the organization of the hindbrain. (A-D) In situ hybridization of anteriorposterior markers in control and MLF-blocked embryos at 24 hpf. (A,B) hoxb1a expression in r4 is unaffected in embryos lacking MLF axons (B) compared with controls (A). (C,D) krox20 expression in r3 and r5 is unaffected in embryos lacking MLF axons (D) compared with controls (C). (E,F) HuC antibody staining in zCREST1:membRFP transgenic embryos at 24 hpf. (F) HuC-labeled neuron number (green) is not affected by blocking MLF axons (F); however, the localization of these neurons is somewhat disorganized compared with controls (E). Dashed lines indicate r5 boundaries. Scale bars: 50 μm for A-D; 20 μm for E,F. |
|
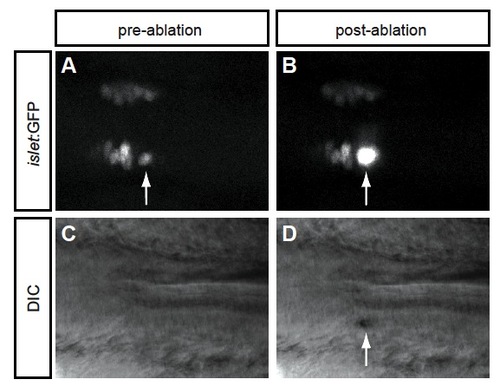
Laser ablation effectively kills the pioneer neuron. (A,B) Dorsal views of a Tg(islet:GFP) embryo (A) before and (B) immediately after ablation of the pioneer neuron (arrow). (C,D) DIC imaging of the same embryo (C) before and (D) immediately after ablation of the pioneer neuron. (B,D) Arrows indicate that the pioneer neuron has indeed been killed by the ablation. |
|
Loss of both Cdh2 and MLF axons in the hindbrain blocks FBMN migration. (A-C) Maximum projection dorsal views of FBMNs (red) and the MLF (green) in 24 hpf Cdh2-depleted Tg(zCREST1:membRFP) zebrafish embryos stained with zn-12 antibody. In all treatments, FBMNs coalesce in the midline; however, FBMNs never migrate along either the LLF or projections of the reticulospinal neurons. Broken lines highlight r5 boundaries. (A) Uncut control Cdh2-depleted embryos at 24 hpf show typical Cdh2-depletion phenotype of FBMN migration into r5 and coalescence of neurons in the midline. In a smaller proportion of Cdh2-depleted embryos, FBMNs still coalesce at the midline but are able to migrate into r6. (B) In embryos lacking both Cdh2 and the MLF, the majority of embryos show FBMNs that migrate only into r5. (C) In a smaller number of embryos lacking both Cdh2 and the MLF, FBMNs migrate into r6. (D) Percentage of embryos affected by Cdh2 depletion and severing manipulations. Cdh2 depletion alone (MO) results in 82% of embryos exhibiting stalled FBMN migration in r5 and 18% with FBMNs migrating into r6. In Cdh2-depleted embryos in which the embryos were severed incompletely so the MLF is still present (MO +MLF), 48% of embryos show stalled migration in r5, whereas 52% of embryos show FBMN migration reaching r6. In embryos depleted of both Cdh2 and the MLF (MO –MLF), 89% result in FBMNs stalling in r5 and 11% result in FBMNs reaching r6. The number of embryos scored is indicated. Scale bar: 20 μm. |