- Title
-
Two types of tet-on transgenic lines for doxycycline-inducible gene expression in zebrafish rod photoreceptors and a gateway-based tet-on toolkit
- Authors
- Campbell, L.J., Willoughby, J.J., and Jensen, A.M.
- Source
- Full text @ PLoS One
|
Generation of a rod-specific, doxycycline-inducible, self-reporting gene expression system. (A) Diagram of the construct used to generate a stable transgenic zebrafish line that expresses doxycycline (Dox)-inducible GFP specifically in rod photoreceptors. The Xenopus rhodopsin promoter (Xla.rho) drives expression of the reverse tetracycline-controlled transcriptional activator (rtTA) gene while the tetracycline responsive element (TRE) drives expression of GFP in the converse direction. (B, C) Confocal z-projections of retinal sections from 6 dpf Tg(Xla.rho:rtTA, TRE:GFP) larvae labeled with anti-Rhodopsin antibody (red). (B) No GFP fluorescence (green) is visible in the absence of Dox. (C) Rod photoreceptors show strong GFP fluorescence (green) when transgenic larvae are treated for 72 h with Dox (3–6 dpf). (D, E) Confocal z-projections of the photoreceptor layer of retinas from Tg(Xop:rtTA, TRE:GFP) adult fish labeled with anti-Rhodopsin (red) and anti-GFP (green) antibodies. (D) No anti-GFP immunofluorescence (green) is visible in the untreated adult photoreceptors, whereas strong anti-GFP immunofluorescence (green) is visible in the adult photoreceptors after treatment with Dox for 72 h (E). dA, polyadenylation signal; Tol2, pTol integration site. Scale bars, 50 µm. |
|
Time course of doxycycline-induced GFP expression in Tg(Xla.rho:rtTA, TRE:GFP) larvae. Confocal z-projections of retinal sections from 6 dpf Tg(Xla.rho:rtTA, TRE:GFP); alb/ larvae that were (A) untreated or treated with doxycycline (Dox) for (B) 1, (C) 2, (D) 4, (E) 8, (F) 16, (G) 24, (H) 48, or (I) 72 h. Anti-Rhodopsin immunofluorescence (red) labels rod photoreceptor outer segments and is visible for all treatment conditions. Anti-GFP immunofluorescence (green) is not visible in the untreated retinal section (A). The number of rods showing anti-GFP immunofluorescence (green) noticeably increases with an increase in the Dox exposure time (B–I). Scale bar (A), 50 µm. |
|
Transactivation of an injected plasmid into Tg(Xop:rtTA, TRE:GFP). (A) Diagram of the tetracycline response element (TRE) construct injected into Tg(Xla.rho:rtTA, TRE:GFP) one-cell embryos. The TRE drives the mCherry gene with a nuclear localization sequence (nls-mCherry). (B–D) Confocal z-projections of retinal sections from injected Tg(Xla.rho:rtTA, TRE:GFP) larvae at 6 dpf that were treated for the final 48 h with doxycycline (Dox) and were labeled with anti-Rhodopsin antibody. (B, B2) nls-mCherry fluorescence (red) is visible in the photoreceptor layer. (C, C2) nls-mCherry fluorescence (red) co-localizes with green fluorescent protein (GFP) fluorescence (green) in the photoreceptor layer. (D, D2) Anti-Rhodopsin label (blue) shows that GFP and nls-mCherry are expressed in rod photoreceptors. Boxed regions in B, C, and D correspond to B2, C2, and D2. dA, polyadenylation sequence; Tol2, pTol integration site. Scale bar, 50 µm. |
|
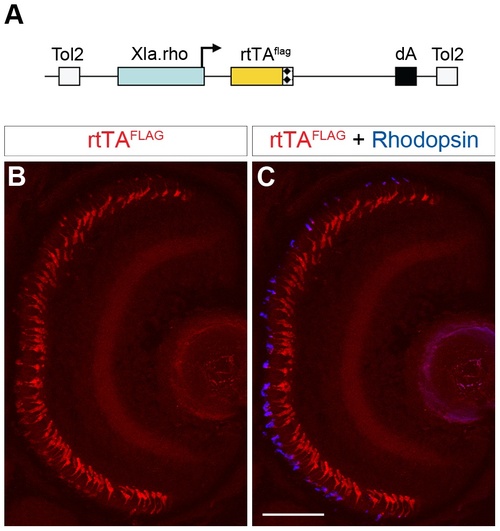
Generation of a rod-specific, epitope-tagged, rtTA-expressing transgenic line for doxycycline-inducible gene expression. (A) Diagram of the construct used to generate a stable transgenic zebrafish line that expresses a FLAG-tagged rtTA protein specifically in rod photoreceptors. The Xenopus rhodopsin promoter (Xla.rho) drives the flag epitope-tagged reverse tetracycline-controlled transcriptional activator (rtTAflag) gene. (B, C) Confocal z-projection of a retinal section from a 6 dpf Tg(Xla.rho:rtTAflag) larva labeled with anti-FLAG and anti-Rhodopsin antibodies. (B) Anti-FLAG immunofluorescence (red) is visible in the photoreceptor layer. (C) The anti-FLAG immunofluorescence (red) colocalizes with the anti-Rhodopsin immunofluorescence (blue) in the rod photoreceptors. dA, polyadenylation sequence; Tol2, pTol integration site. Scale bar, 50 µm. |
|
Bidirectional transactivation of an injected biTRE-containing plasmid into Tg(Xla.rho:rtTAflag). (A) Diagram of the bidirectional tetracycline response element (biTRE)-containing construct injected into Tg(Xla.rho:rtTAflag) one-cell embryos. EGFP and mCherry with a nuclear localization sequence (nls-mCherry) flank the biTRE. (B–G) Confocal z-projections of retinal sections from injected Tg(Xla.rho:rtTAflag) larvae at 6 dpf labeled with anti-FLAG antibody (blue). (B–D) GFP fluorescence (B, green) and nls-mCherry fluorescence (C, red) are undetectable in the absence of doxycycline (Dox) treatment, while anti-FLAG labeling (D, blue) is visible in rod photoreceptors. (E–G) GFP fluorescence (E, E2, green) and nls-mCherry fluorescence (F, F2, red) are visible in the photoreceptor layer and co-localize with anti-FLAG labeling (G, G2, blue) in the rod photoreceptors after 48 h Dox treatment. Boxed regions in E, F, and G correspond to E2, F2, and G2. dA, polyadenylation sequence; Tol2, pTol integration site. Scale bar (G), 50 µm. |
|
Transactivation of the transgenic TRE:HA-Crb2aIntraWT. (A) Larvae from an outcross of Tg(Xla.rho:rtTA, TRE:GFP) to Tg(TRE:HA-Crb2aIntraWT) were untreated or doxycycline (Dox)-treated for 72 hours and genotyped for the transgenes before immunofluorescence analysis. (B–J) Confocal z-projections of retinal sections from 6 dpf Tg(Xla.rho:rtTA, TRE:GFP; TRE:HA-Crb2aIntraWT) larvae labeled with anti-HA (red) and anti-Rhodopsin (blue) antibodies. (B–D) Anti-HA-Crb2aIntraWT immunofluorescence (B, red) and GFP fluorescence (C, green) are undetectable in the untreated rod photoreceptors with anti-Rhodopsin immunofluorescence (D, blue). (E–J) Anti-HA-Crb2aIntraWT immunofluorescence (E, H, red) and GFP fluorescence (F, I, green) are visible in the photoreceptor layer and co-localize with the anti-Rhodopsin immunofluorescence in the rod photoreceptors after 72 h of Dox treatment. Scale bar (D, G), 50 µm and (J), 20 µm. |