- Title
-
In vitro oocyte culture-based manipulation of zebrafish maternal genes
- Authors
- Nair, S., Lindeman, R.E., and Pelegri, F.
- Source
- Full text @ Dev. Dyn.
|
Oogenesis occurs predictably after purging and oocytes of all stages are translation competent. Live images of oocytes. A–P: Oocytes immediately after isolation from purged females on the indicated dpp at time 0 (A,E,I,M) and 2 hr after GFP mRNA injection (B,F,J,N), expressing green fluorescent protein (GFP) protein (C,D,G,H,K,L,O,P). B,F,I,J: Injected Stage I and II (B) and early III (F) oocytes degenerate, while late stage III oocytes (I) mature into stage IV transluscent oocytes (J) with germinal vesicle (arrow). Injected Stage IV oocytes (M) containing germinal vesicle (arrow) mature into stage V oocytes (N, note germinal vesicle breakdown). Scale bar = 300 μm in A–P. |
|
Wild-type aurB mRNA injected into stage IV cei/aurB oocytes rescues the maternal-effect phenotype in cei/aurB embryos. A–L: Animal views of 65 mpf blastodiscs immunolabeled to detect α-tubulin (A,E,I) and β-catenin (B,F,J), with DAPI (4′,6-diamidine-2-phenylidole-dihydrochloride) stainings (C,G,K) and panel merges (D,H,L). A,B,E,F: Wild-type embryos show robust furrows between cells and β-catenin accumulation at the furrows (A,B), while cei/aurB embryo from uninjected cei/aurB stage IV oocyte shows partial, rudimentary furrows (E), which do not accumulate β-catenin (F). I,J: Rescued cei/aurB embryo from cei/aurB oocyte injected with wild-type aurB mRNA labeled with α-tubulin (I) show robust furrows which accumulate β-catenin (J), indicating furrow maturation. C,G,K: DAPI images show that nuclear division proceeds normally in all cases. |
|
Stage IV oocyte injection of aurB-KD mRNA phenocopies the cei/aurB maternal-effect phenotype. A–L: Animal views of 65 mpf blastodiscs immunolabeled to detect α-tubulin (A,E,I) and β-catenin (B,F,J), with DAPI (4′,6-diamidine-2-phenylidole-dihydrochloride) stainings (C,G,K) and panel merges (D,H,L). A,B: Robust furrows in wild-type embryos labeled with α-tubulin (A), which accumulate β-catenin (B). E,F: &alphja;-Tubulin labeling of control cei/aurB embryos from mutant stage IV oocytes show partial rudimentary furrows (E) with no β-catenin labeling (F). I,J: α-tubulin labeled partial, immature furrows in embryos from wild-type stage IV oocytes injected with aurB-KD mRNA (I), which also fail to accumulate β-catenin (J), recapitulating cei/aurB maternal effect phenotype. C,G,K: As in Figure 2, DAPI images show that nuclear division proceeds normally in all cases. |
|
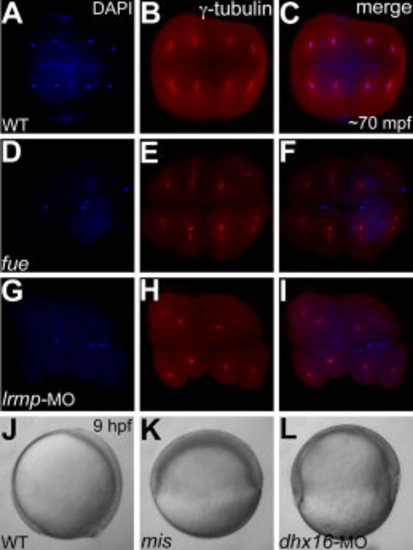
Stage IV oocyte injections of Lrmp and Dhx16 morpholinos phenocopy fue and mis maternal-effect phenotypes, respectively. A–I: Animal views of immunolabeled 70 minutes postfertilization (mpf) blastodiscs stained for DAPI (A,D,G) immunolabeled to detect γ-tubulin (B,E,H) and panel merges (C,F,I). A–C: In wild-type embryos, each nucleus associates with centrosomal γ-tubulin staining. D–F: In maternal-effect fue mutants, nuclei fail to divide, resulting in two to three patches of nuclei stainings corresponding to unfused parental pronuclei and the polar body for meiosis II (D,F), which fail to associate with γ-tubulin (E,F). G–I: In Lrmp morphants where maternal Lrmp function was inhibited, the nuclei similarly fail to divide (G) and also fail to associate with γ-tubulin (H,I). J–L: Lateral views of live embryos at 9 hours postfertilization (hpf). J,K: Wild-type embryos at 9 hpf where axis formation is evident (anterior to the top and dorsal to the right) (J), while in maternal-effect mis mutants, epiboly fails to occur (K). L: In Dhx16 morphants where maternal function was inhibited, epiboly similarly arrests, resulting in gastrulation failure. |
|
Sass6-mCherry protein from exogenous mRNA injected into stage IV oocytes localizes to centrioles during early cleavage stages. Immunolabeling for γ-tubulin and DAPI staining for nuclei in a Sass6-mCherry expressing blastodisc during prophase (40 mpf), which contains two nuclei, both of which are shown. C,E,H,J: γ-tubulin labels pericentriolar material around the nuclei, with intense foci in presumptive centrioles flanking each nuclei (arrows). B,D,E,G,I,J: Ectopically expressed Sass6-mCherry protein also localizes to foci flanking the nuclei (arrows in B,D,G,I) and colocalizes with γ-tubulin foci (arrows in E,J). B,G: Ectopic Sass6-mCherry is also observed as additional foci near the nucleus periphery (white arrowheads in B,G) and in the cytoplasm (not shown), consistent with the production of supernumerary centrioles after overexpression of maternally-derived Dsas-6 in Drosophila embryos (Peel et al., 2007). G,J: The observed putative supernumerary foci can also be associated with extra γ-tubulin foci (yellow arrowhead). Merge1: DAPI and Sass6::mCherry; merge2: DAPI, Sass6::mCherry and γ-tubulin. |
|
Mature mRNAs are present during very early stages of oogenesis. RT-PCRs for exonic (A) and intronic (B) transcripts for β-actin and sass6 from whole ovaries day 1 post-purging (lanes 1, 2, 7, 8) and from stage IV oocytes (lanes 4, 5, 10, 11). Mature mRNAs for β-actin and sass6 isolated by oligodT priming are present in ovaries at day 1 post-purging (lanes 1, 2) and in stage IV oocytes (lanes 4, 5). Pre-spliced immature mRNAs for β-actin and sass6 isolated by random hexamer priming are also present in ovaries at day 1 post-purging (lanes 7, 8) and in stage IV oocytes (lanes 10, 11). For the negative controls (lanes 3, 6, 9 12) the respective total RNA samples was used as template in the PCR reaction with β-actin primers, to confirm that the RNA used for cDNA synthesis was free of genomic DNA. |