- Title
-
The role of microtubules in neutrophil polarity and migration in live zebrafish
- Authors
- Yoo, S.K., Lam, P.Y., Eichelberg, M.R., Zasadil, L., Bement, W.M., and Huttenlocher, A.
- Source
- Full text @ J. Cell Sci.
|
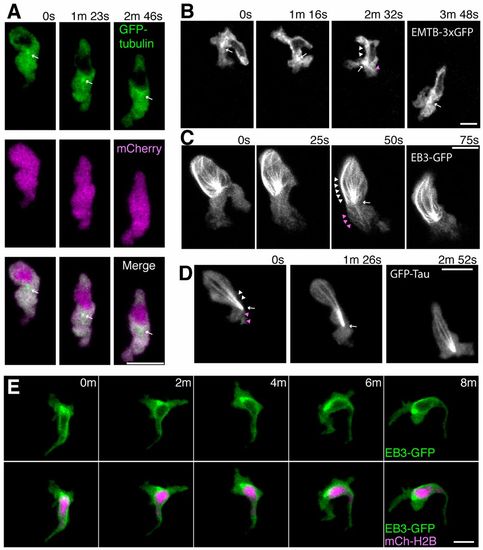
Localization of microtubules during neutrophil motility in live zebrafish. (A) Time-lapse imaging of a neutrophil expressing GFP-tubulin and mCherry. Arrows indicate a punctate dot in front of the nucleus. Note that fluorescent signals of GFP-tubulin are excluded from the nucleus. (B) Time-lapse imaging of a neutrophil expressing EMTB-3xGFP. A punctate dot, presumably the MTOC, is indicated by arrows. Arrowheads indicate linear structures (presumably microtubules) extending to the leading edge (magenta) and to the uropod (white). (C) Time-lapse imaging of a neutrophil expressing EB3-GFP. An arrow indicates the MTOC and arrowheads indicate microtubules extending to the leading edge (magenta) and to the uropod (white). (D) Time-lapse imaging of a neutrophil expressing GFP-Tau. An arrow indicates the MTOC and arrowheads indicate microtubules extending to the leading edge (magenta) and to the uropod (white). (E) Time-lapse imaging of a neutrophil expressing EB3-GFP and a nucleus probe mCherry-histone H2B. Data are representative of more than three separate time-lapse movies. Scale bars: 10 μm. |
|
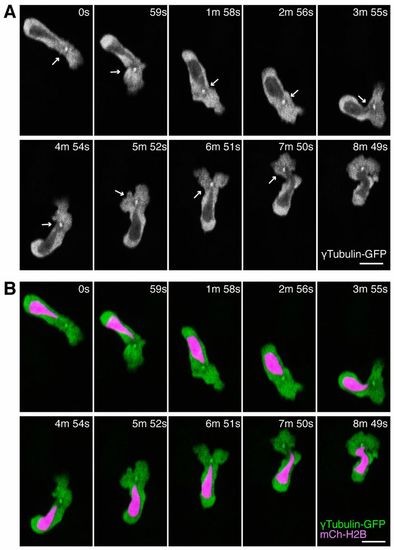
The MTOC is localized in front of the nucleus during neutrophil motility in live zebrafish. (A) Time-lapse imaging of a neutrophil expressing tubulin-GFP. Arrows indicate the MTOC in front of the nucleus. (B) Simultaneous imaging of tubulin-GFP and a nucleus probe mCherry-histone H2B in the same cell with A. Data are representative of more than three separate time-lapse movies. Scale bars: 10 μm. |
|
Microtubule depolymerization impairs neutrophil wound attraction but enhances neutrophil motility and F-actin polarity. (A) Neutrophil recruitment to wounded fins at 30minutes post wounding. Neutrophils were stained with Sudan Black. n = 42 (DMSO), 22 (Noc 0.5μM) and 22 (Noc 2&mu′M). (B–D) Microtubule depolymerization with nocodazole treatment enhances neutrophil 3D motility [(B), n = 32 (DMSO), n = 38 (Noc)], makes cells compact [(C), n = 40 (DMSO), n = 48 (Noc)] and induces a more round morphology [(D), n = 40 (DMSO), n = 48 (Noc)]. Tg(mpx:GFP-UtrCH) was used for live analysis. (E) Nocodazole treatment enhances polarity of F-actin dynamics in neutrophils. Rear localization of stable F-actin detected by GFP-UtrCH is particularly emphasized after microtubule depolymerization. Tg(mpx:GFP-UtrCH) was crossed with Tg(mpx:Lifeact-Ruby). *P<0.05, one-way ANOVA with Dunnett post-test (A) and two-tailed unpaired t-test (B–D). Data in A are representative of at least three separate experiments and three time-lapse movies were analyzed for data in B–D. Scale bar: 10 μm. |
|
Microtubule disassembly inhibits PI(3)K activation at the leading edge. (A) Time-lapse ratiometric imaging (PHAKT-GFP/mCherry) of PI(3,4,5)P3–PI(3,4)P2 dynamics during neutrophil random motility in the head. (B) Microtubule depolymerization inhibits localization of PI(3,4,5)P3–PI(3,4)P2 at the leading edge. Scale bars: 10 μm. |
|
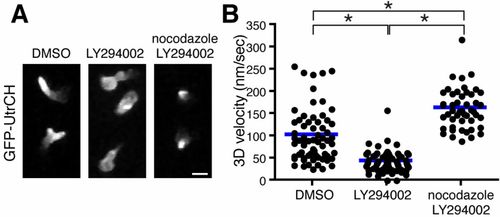
Microtubule disassembly induces neutrophil polarity and motility in a PI(3)K-independent manner. (A) Microtubule depolymerization with nocodazole reverses F-actin polarity defects caused by PI(3)K inhibition. (B) Microtubule disassembly reverses PI(3)K inhibition-induced migration arrest and enhances neutrophil motility compared with control. n = 66 (DMSO), 64 (LY294002) and 48 (nocodazole, LY294002). *P<0.05, one-way ANOVA with Tukey post-test. Three time-lapse movies were analyzed for data for (B). Scale bar: 10 μm. |
|
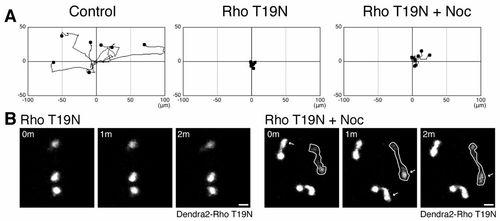
Rho regulates microtubule disassembly-mediated neutrophil motility. (A) Expression of a dominant negative RhoA T19N prevents microtubule depolymerization-induced neutrophil motility. Dendra2-Rho T19N was expressed in Tg(mpx:Lifeact-Ruby) and neutrophil motility was tracked for 30minutes. (B) Nocodazole induces protrusion in Rho-inhibited cells. Data in A are representative of at least three time-lapse movies. Scale bars: 10 μm. |
|
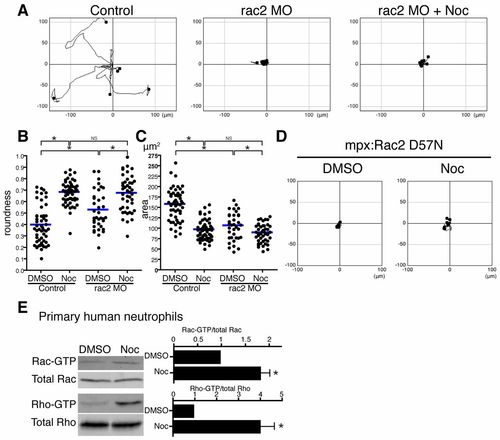
Rac regulates microtubule disassembly-mediated neutrophil motility. (A) rac2 MO inhibits microtubule depolymerization-induced neutrophil motility. Neutrophil motility was tracked for 30minutes in Tg(mpx:GFP-UtrCH). (B) Rac2 knockdown makes neutrophils round, but nocodazole treatment makes Rac2-inhibited cells more round. n = 53 (Control, DMSO), 52 (Control, Noc), 36 (rac2 MO, DMSO) and 44 (rac2 MO, Noc). (C) Rac2 knockdown makes neutrophils compact, but nocodazole treatment makes Rac2-inhibited cells more compact. n = 54 (Control, DMSO), 52 (Control, Noc), 36 (rac2 MO, DMSO) and 44 (rac2 MO, Noc). (D) Transgenic neutrophil specific expression of dominant negative Rac2 D57N inhibits microtubule depolymerization-induced neutrophil motility. Neutrophil motility was tracked for 30minutes in Tg(mpx:mCherry-2A-Rac2D57N). (E) Affinity precipitation assays with GST-RBD and GST-PBD to detect Rho-GTP and Rac-GTP, respectively (n = 3). Microtubule disassembly directly activates Rac in addition to Rho in primary human neutrophils. Data in A and D are representative of at least three time-lapse movies. Three time-lapse movies were analyzed for data for B and C. Data in E are means ± s.e.m. of three separate experiments. *P<0.05, one-way ANOVA with Bonferroni post-test (B,C) and two-tailed paired t-test (E). |
|
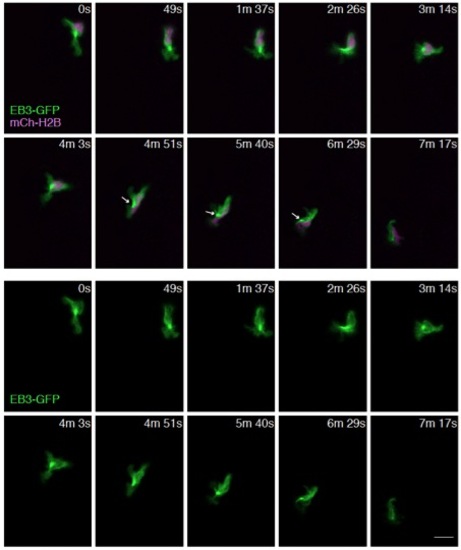
The MTOC is mainly localized in front of the nucleus and occasionally on the side of the nucleus. Time-lapse imaging of a neutrophil expressing EB3-GFP and a nucleus probe mCherry-histone H2B. Arrows indicate localization of MTOC on the side of the nucleus. Scale bar: 10 μm. |