- Title
-
Neutrophil-Delivered Myeloperoxidase Dampens the Hydrogen Peroxide Burst after Tissue Wounding in Zebrafish
- Authors
- Pase, L., Layton, J.E., Wittmann, C., Ellett, F., Nowell, C.J., Reyes-Aldasoro, C.C., Varma, S., Rogers, K.L., Hall, C.J., Keightley, M.C., Crosier, P.S., Grabher, C., Heath, J.K., Renshaw, S.A., and Lieschke, G.J.
- Source
- Full text @ Curr. Biol.
|
Leukocytes Are Required for the Initial Decline in Wound Margin H2O2 Concentrations(A) (i) Mean HyPer ratios reflecting the wound zone [H2O2] profile of WT (injured and uninjured), cloche mutant (clo) and spi1/csf3r morphant embryos. Note the significantly sustained, elevated HyPer ratio/[H2O2] in leukocyte-deficient clo and spi1/csf3r morphants. Insets (ii, iii) show the individual median HyPer data and p values (natural permutation test). The n values are as follows (embryos/independent days/scans): injured WT, 5/2/4; spi1/csf3r morphants, 4/2/3; clo 6/3/5; uninjured WT, 5/1/5.(B) HyPer heat maps depicting [H2O2] across the wounded tail fin at five sequential time points. Accompanying overlaid transmission (Trans) and DsRED2 images demonstrate number and position of Tg(lyz:DsRED2) neutrophils. Note the single DsRED2-positive cell in the spi1/csf3r morphant (black arrow head). clo did not carry the lyz:DsRED2 transgene. Scale bar (applies to all panels) represents 200 μm. Heat map legend applies to all figures. The stills are from Movie S1. PHENOTYPE:
|
|
Myeloperoxidase (mpx) Deficiency in the Zebrafish Mutant durif (drf)(A) mpx expression by whole-mount in situ hybridization (WISH) in WT and drf embryos.(B and C) Loss of cells carrying Mpx activity in drf demonstrated by loss of Mpx-dependent histochemical staining (B) and stable sudanophilia (C) (arrowheads indicate positive cells in WT).(D) Cytospun adult kidney hematopoietic cells, stained for Mpx by diaminobenzidine (DAB) histochemistry with May-Grunwald/Giemsa counterstain. WT but not drf neutrophils show Mpx staining (black triangles). Providing negative and positive internal staining controls, both genotypes have Mpx-negative eosinophils (black arrow) and weak erythrocyte staining due to the pseudoperoxidase Lepehne reaction (open triangles). Scale bar represents 10 μm.(E) Ultrastructure of adult WT and drf neutrophils, showing normal primary granules. Scale bars represent left, 2 μm; right, 100 nm.(F) drf-/- Tg(mpx:EGFP) hemizygotes have normal numbers of EGFP-expressing cells (mean ± SE: WT, 120 ± 6, n = 16; drf, 116 ± 5, n = 15).(G) Positional cloning placed drf on chromosome 10 within 0.08 cM of RFLP-356k located ~1.5 kb 3′ from the mpx gene.(H) Schematic of the mpx locus (black, exon; white, UTR; based on GenBank BC068379.1). Left sequencing chromatograms of complementary DNA shows the exon 11/12 junction: a 7 nt insertion in drf introduces a premature stop codon (*). Right sequencing chromatograms of genomic DNA show the drf mutation (red arrow), a T→A transversion 9 nt 52 of the intron 12/exon 13 junction. |
|
Neutrophil-Delivered Myeloperoxidase Is Required for the Initial Decline in Wound Margin H2O2 Concentrations(A) (i) Mean HyPer ratios reflecting the wound zone [H2O2] data for injured and uninjured drf embryos. Note the sustained, elevated HyPer ratio/[H2O2] in mpx-deficient drf mutants. These drf data are directly comparable with data of Figure 1A and share its WT control group. Inset (ii) shows the individual median HyPer data and p value compared to WT (natural permutation test). The n values are as follows (embryos/independent days/scans): injured drf, 6/5/6; uninjured drf, 4/1/4. (iii) Representative HyPer heat maps depicting [H2O2] across the wounded tail fin of an mpx-deficient drf embryo with overlaid transmission (Trans) and DsRED2 images demonstrating the proximity of neutrophils to the wound margin. Scale bar represents 200 μm. Stills are from Movie S1 at same time points as in Figure 1B.(B) (i) Transplantation strategy to generate HyPer-mRNA-loaded, Mpx-deficient, drf embryos selectively populated with DsRED2-expressing WT neutrophils. (ii) A successful transplant outcome. Brightfield image of 3 dpf embryo with superimposed green and red fluorescence images, showing a single residual recipient-derived EGFP-expressing neutrophil (green arrow) and scattered DsRED2-expressing donor neutrophils (red arrows).(C) (i) Mean HyPer ratios reflecting the wound zone [H2O2] profile of drf embryos carrying WT neutrophils, which arrive (pink) or do not arrive (orange) at the wound. WT neutrophil arrival recapitulates the WT HyPer ratio profile; WT cell nonarrival serves as a negative control demonstrating the sustained [H2O2] elevation of a nonrescued Mpx-deficient drf profile. Inset (ii) shows the individual median HyPer data. The n values are as follows (embryos/independent days/scans): WT cells arrived, 3/3/3;WT cell did not arrive, 3/3/3.(D) HyPer heat maps depicting [H2O2] across the wounded tail fin of a transplanted drf embryo with arriving WT cells, demonstrating lower [H2O2] levels in the tail half where WT DsRED2-expressing cells arrive. Overlaid transmission (Trans) and DsRED2 images demonstrate the proximity of Tg(lyz:DsRED2) neutrophils to the wound margin. Scale bar (applies to all panels) represents 200 μm. PHENOTYPE:
|
|
Myeloperoxidase and H2O2 Interact Directly within Neutrophils at the Wound Zone(A) HyPer480 images showing neutrophil-specific HyPer expression in Tg(lyz:HyPer) embryos, with HyPer ratio heat maps reflecting the corresponding intraneutrophil [H2O2] levels at four time points after wounding. Stills from Movie S3. Corresponding graphs show single neutrophil mean HyPer ratios plotted against distance from wound for four individual embryos, with individual animal regression lines (individual animals color-coded). The negative gradient at 30–50 min after wounding contrasts with the uniform intraneutrophil [H2O2] levels prior and later, and indicates higher internal [H2O2] within wound-proximate neutrophils at these time points.(B–F) Demonstration of a direct enzymatic Mpx/H2O2 interaction within the wound zone by histochemical staining for Mpx activity. For each reaction condition (B–F), the three panels are (i) an overview, (ii) details of cells in the caudal hematopoietic tissue, and (iii) wound zone. Stained cells (white arrows) indicate Mpx-dependent catalysis of p-phenylenediamine and catechol. When H2O2 is supplied as a reagent, cells stain throughout the embryo (B), but without supplemented H2O2, cells stain only at the wound margin (C). (D)–(F) are negative controls: (D) and (E) drf controls show that staining is mpx-dependent regardless of the H2O2 source; (F) WT uninjured control showing that detecting staining of wound-proximate cells is injury-dependent. x/y n values are as follows: (embryos with e1 positive cell in region)/(embryos scored).(G–I) The relative [H2O2] within and surrounding wound zone neutrophils, demonstrated using HyPer-mRNA-loaded Tg(lyz:HyPer) embryos. (G) Groups of neutrophils 29 and 85 min after wounding displayed by their HyPer480 signal, with corresponding HyPer ratio images portraying intra- and extracellular [H2O2]. At 29 min, all neutrophils have higher [H2O2] internally than externally, but at 85 min, a cluster of 3 neutrophils displays lower [H2O2] internally than externally. Stills from Movie S4 are shown. (H) Tracks of nine neutrophils plotted in space (x,y) and time (z), with tracks colored in categories to indicate when the neutrophil internal [H2O2] exceeds (green), equals (blue), or is less than (red) the external [H2O2]. Legend gives percentage of exterior HyPer ratio category cut-offs. The nine cells are three neutrophils (C1-3) selected from three wounded embryos (W1–W3). (I) The difference between internal and external [H2O2] in time for these same nine cells expressed as the difference between internal and external mean HyPer ratios. Six out of nine cells in (H, I) are net [H2O2] consumers, and in 5/6 cases this phenotype is accentuated as they approach the wound margin (one cell does not migrate). Scale bars in (A) and (G) represent 50 μm. Unlabelled perspective-shortened y axis marks (H, left upper corner) indicate 50 μm steps. |
|
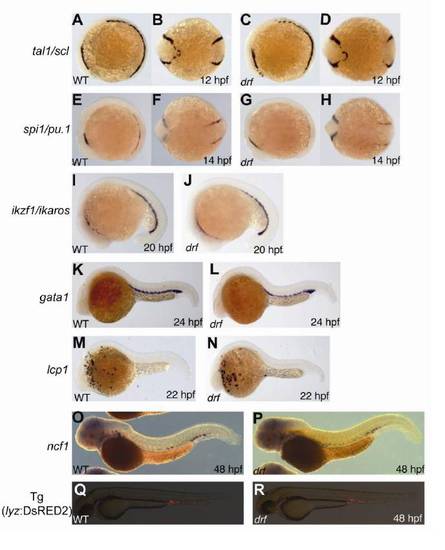
Hematopoietic Gene and Marker Gene Expression in the myeloperoxidase-Deficient Zebrafish Mutant durif, Related to Figure 2 |
|
Zonal Variations in Tissue [H2O2] Related to the Path of an Individual Neutrophil Migrating towards the Wound Zone, Related to Figure 4, and a Model of the H2O2/Myeloperoxidase Interaction after Wounding |