- Title
-
Scube activity is necessary for Hedgehog signal transduction in vivo
- Authors
- Johnson, J.L., Hall, T.E., Dyson, J.M., Sonntag, C., Ayers, K., Berger, S., Gautier, P., Mitchell, C., Hollway, G.E., and Currie, P.D.
- Source
- Full text @ Dev. Biol.
|
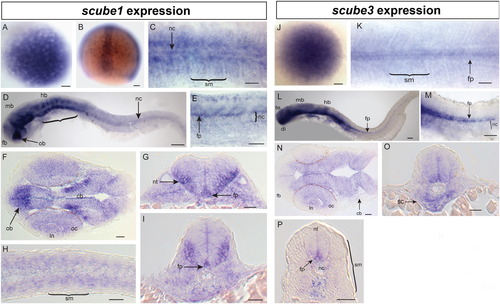
mRNA expression patterns of scube1 and scube3. (A–I) scube1 mRNA expression. (A) Maternal expression of scube1 in 256 cell embryo, (B) scube1 is expressed in a stripe of cells corresponding to the developing notochord (nc) at <90% epiboly. (C) At 15 somites, scube1 is expressed in the somitic mesoderm (sm), and notochord. (D, E) At 26 somites scube1 expression is in the forebrain (fb), olfactory bulb (ob), midbrain (mb), hindbrain (hb), rhombomeres (bracket), with diffuse staining present in notochord and floorplate (fp) of neural tube [E closeup of axial somites in embryo equivalent to D]. (F–I) Sections of 24 hpf embryos. (F) scube1 expression in forebrain, cerebellar neurons (cb), lens (ln), optic cup (oc) with strong expression in the olfactory (ol) forebrain region. (G, I) scube1 is expressed throughout the neural tube (nt) with strong floorplate and lateral nt expression. (H, I) scube1 somitic mesoderm expression in tail sections. (J–P) scube3 mRNA expression. (J) Maternal expression of scube3 in 256 cell embryo. (K) scube3 expression in the floorplate, notochord, somitic mesoderm, and PSM at 15 somites. (L, M) Extensive neural expression (telencephalon te, diencephalon, di, midbraim, mb, and hindbrain, hb), expression in the floorplate, somites and diffusely in the notochord at 26 somites. (M) Magnification of tail region in (L) showing strong ventral floorplate staining and weak notochord staining. N–P sections of 24 hpf embryos. (N) scube3 is expressed in the cerebellum, the lens of the eye and the optic cup and weakly in the forebrain. (O) scube3 expression is strong in sclerotome ventral to the notochord. (P) scube3 expression throughout neural tube, strong expression in floorplate and weak notochord expression. Scale Bars: 100 μm. (C, K) Dorsal view, (D, E, L, M) lateral view (F, H, N) longitudinal sections, (G, I, O, P) transverse sections. |
|
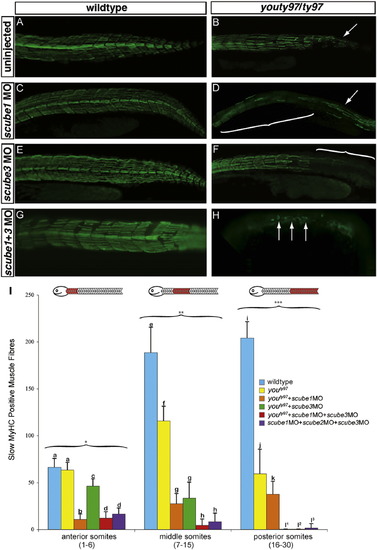
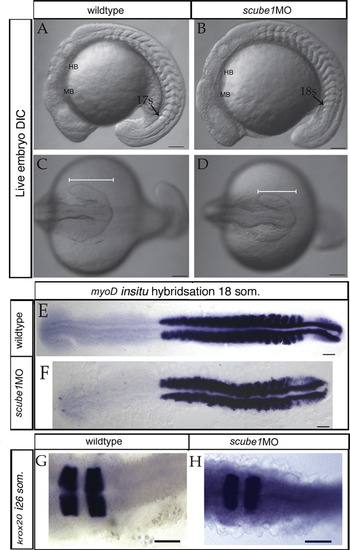
scube1 and scube3 morpholinos enhance the phenotype of youty97 mutant embryos. The wildtype pattern of slow MyHC (A) is disrupted in the posterior tail region of youty97 homozygotes (B) (arrow). The pattern of slow MyHC is not visibly affected when scube1MO (C) scube3MO (E) or both scube1MO+scube3MO (G) are injected into wildtype embryos. (D) scube1MO injected into youty97 homozygotes enhanced the youty97 phenotype. Slow MyHC expression is disrupted after PHENOTYPE:
|
|
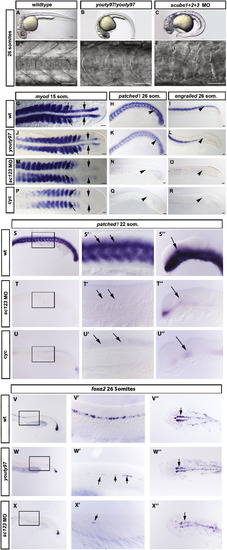
scube1 and scube3 morpholinos enhance the phenotype of youty97 mutant embryos. (A–F). Somite shape in live 30 hpf embryos, lateral view anterior to left. (A, D) The characteristic chevron shape of the wildtype zebrafish somite became rounded or ‘U’ shaped in the youty97 mutant (B, E). (C, F) Morpholino knockdown of all 3 scube genes enhanced the youty97 mutant phenotype, a severe loss of the chevron shape resulted in ‘blocky’ shaped somites. Scale Bars: A–C 150 μm, D–F 100 μm. (G–R). In situ hybridization revealed Hh target gene expression (myod, patched1 (ptc1), and engrailed (eng)), decreased dramatically in scube triple MO embryos. (G) myod is expressed in somites and adaxial cells of wildtype embryos, (J) adaxial expression of myod is reduced in youty97 homozygous mutants, (M) adaxial myod expression is absent from scube triple MO embryo. (P) Adaxial myod expression is also absent from cyclopamine treated embryos. (G, J, M, P) Arrows indicate location of adaxial cells. (H) ptc expression in ventral neural tube and somites in wildtype embryos. (K) Reduced ptc expression in posterior somites of youty97 embryo, (N) somitic expression of ptc is absent from scube triple MO and (Q) cyclopamine treated embryos. (H, K, N, Q) Arrowheads highlight regions of altered gene expression. (I) In wildtype embryos eng is expressed in the muscle pioneer cells and a subset of fast muscle fibers, (L) eng expression is reduced in the posterior of youty97 mutant embryos, (O) in scube triple MO embryos eng expression is severely reduced with only small patches of eng positive cells visible. (R) Cyclopamine treated embryos display the most severe loss of eng staining. (I, L, O, R) Arrowheads highlight regions of altered gene expression. (G, J, M, P) 15 somite stage, dorsal view, (H, K, N, Q) 22 somite stage, lateral view anterior to left, (I, L, O, R) 26 somite stage, lateral view, anterior to left. Scale bars: 50 μm. (S–U′′) ptc1 expression is reduced in neural tissue of scube triple MO embryos. In wildtype, uninjected 22 somite embryos (S–S′′), ptc1 expression is prominent in the ventral neural tube of the trunk (S and arrows in S′) as well as the somites, where its expression is known to be induced by HH signaling from the midline. In the brain, ptc1 is highly expressed within the diencephalon (S′′, arrow), an expression domain known to be induced by HH signaling from the underlying zona limitans intrathalamica. In both scube triple MO embryos (T–T′′) and in cyclopamine treated embryos (U–U′′) expression of ptc1 in both these tissues is severely reduced or absent. (V–X′′) Expression of the ventral neural marker foxa2 is severely reduced in scube triple MO embryos. In wildtype embryos at 26 somites foxa2 is expressed in floor plate (V, V′ both medial and lateral cells) and in two wings of expression in the ventral diencephalon (arrow, V′). In homozygous youty97 mutants both these regions of expression are reduced (Fig. 3W–W′′), and in scube triple MO embryos this reduced expression is greatly enhanced (X–X3). (S–X′′) Within each row, the same letter indicates a different region of the same embryo.′ Add –(S–X′′) anterior to left, (S–U′′) 22 somites, lateral view (V,V′, W, W′, X, X′) 26 somites, lateral view, (V′′, W′′, X′′) dorsal view. EXPRESSION / LABELING:
PHENOTYPE:
|
|
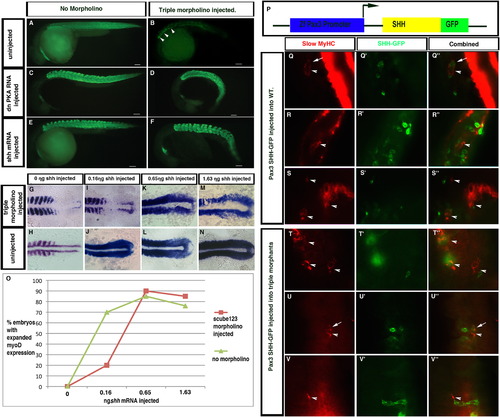
Epistatic analysis of scube function in the Hedgehog pathway. Hedgehog pathway overexpression induces ectopic slow MyHC expression in zebrafish embryos. (A–F). Slow MyHC expression in treated and untreated embryos. (A) Slow MyHC localization in wildtype embryos. (B) scube triple MO embryos show severe reductions in slow MyHC expression. Representative embryo (B) has 15 slow MyHC positive fibers (arrowheads). (C) Overexpression of dnPKA RNA turns all muscle in the embryo into slow muscle. (D) Overexpression of dnPKA in scube triple MO embryos rescued the loss of slow muscle seen in scube triple MO embryos. (E) shh RNA overexpression induced ectopic slow MyHC expression in wildtype embryos. (F) shh RNA overexpression in scube triple MO embryos rescued the loss of slow muscle in scube triple MO embryos. Lateral views of<30 hpf embryos. F59 monoclonal antibody labels slow MyHC positive muscle fibers, Alexa 488 conjugated-donkey anti-mouse secondary antibody, 0.65 ·g of dnPKA RNA and shh RNA was injected at 100 μg/mL. Scale Bars: 100 μm. (G–O) Titration of shh RNA in scube triple MO embryos (G, I, K, M) and uninjected 10 somite embryos (H, J, L, N). Expansion of myod expression is seen at 0.16 ng in embryos not injected with Scube morpholinos (J) compared to 0.65 ng in scube triple MO embryos (K). (O) Graph showing percentage of embryos with expanded myod expression. (P–V3) Mosaic analysis of cells expressing SHH fused to GFP under the control of the pax3a promoter. Mosaic expression of pax3a:Shh-GFP generated ectopic slow muscle both cell autonomously (arrows) and non-cell autonomously (arrowheads) in both wildtype (Q–S′′) and scube triple MO embryos (T–V′′). (Q, R, S, T, U, V) F59 monoclonal antibody, (Q′, R′, S′, T′, U′, V′) anti-GFP antibody, (Q′′, R′′, S′′, T′′, U′′, V′′) merged images. |
|
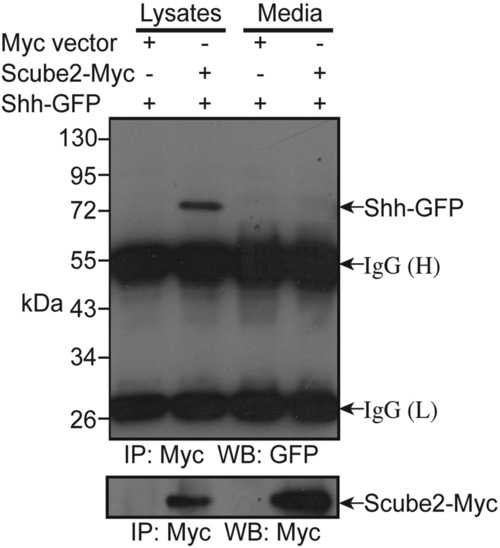
Zebrafish Scube2 binds SHH when co-expressed in COS-1 cells. Binding of Scube2-Myc to SHH-GFP occurs only in the membrane fraction. COS-1 cells were transiently transfected with Scube2-Myc and Shh-GFP. As a control, an empty Myc vector was co-transfected with SHH-GFP. Immunoprecipitation (IP) was performed on lysates and media fractions using anti-Myc antibody. Western blotting (WB) of immunoprecipitated proteins was performed using anti-GFP (upper panel) or anti-Myc (lower panel) antibodies. |
|
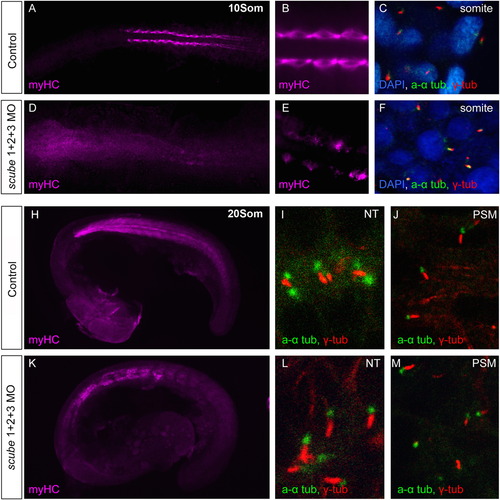
Scube activity is not required for primary cilia formation. (A–M) Scube triple MO injections do not affect formation of primary cilia. (A–C). Water injected control 10 somite stage embryos display normal slow muscle development visualized by the slow myHC specific antibody F59. (A, B) Primary cilia are present in the somites, marked by acetylated alpha (a α)-tubulin (green) and gamma (γ)-tubulin (red) (Nuclei marked with DAPI) (C). (D–F). 10 somite stage embryos injected with scube triple MO have disrupted slow muscle myogenesis (D, E), yet appear to possess normal primary cilia (F). (H–J). In control 20 somite embryos (H) slow myHC staining shows a well-organised myotome with chevron shaped somites. (I) In wildtype embryos, cilia are visible in the neural tube (NT) and presomitic mesoderm (PSM) (J). (K–M). In scube triple MO embryos at 20 somites, myogenesis is disrupted, with somites displaying a U-shaped morphology (K). Cilia visible in the neural tube (L) and paraxial mesoderm (M) appear normal in scube triple MO embryos. PHENOTYPE:
|
|
PHENOTYPE:
|

Unillustrated author statements |
Reprinted from Developmental Biology, 368(2), Johnson, J.L., Hall, T.E., Dyson, J.M., Sonntag, C., Ayers, K., Berger, S., Gautier, P., Mitchell, C., Hollway, G.E., and Currie, P.D., Scube activity is necessary for Hedgehog signal transduction in vivo, 193-202, Copyright (2012) with permission from Elsevier. Full text @ Dev. Biol.