- Title
-
A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting
- Authors
- Hugo, S.E., Cruz-Garcia, L., Karanth, S., Anderson, R.M., Stainier, D.Y., and Schlegel, A.
- Source
- Full text @ Genes & Dev.
|
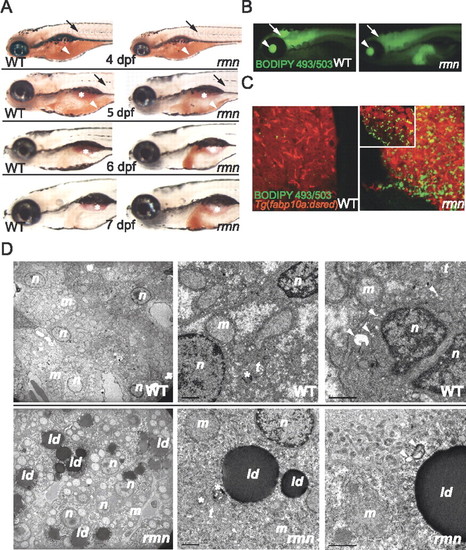
Identification of the fasting hepatic steatosis mutant rmn. (A) Whole-mount ORO staining of larvae. The yolk lipid (arrowhead) is exhausted 5 dpf. Vascular lipid staining (arrows) ceases by the end of 5 dpf. The surfactant-lined swim bladder stained with ORO (asterisk). (B) Whole-mount BODIPY 493/503 staining of 6-dpf larvae. This dye also stained the vitreous humor of the eyes (arrowhead) and the ventricles of the CNS (arrow). (C) Confocal stacks of livers fixed and stained as in B. A single slice of a rmn mutant liver is shown in the inset. (D) Transmission electron microscopy of liver sections showing that the rmn mutant hepatocytes have cytoplasmic lipid droplets (ld). The nuclear (n) and mitochondrial (m) morphology appears normal in rmn mutants. Higher-magnification micrographs also show multilamellar structures (asterisks) suggestive of multivesicular bodies and elongated tubular (t) precursors of these structures in both wild-type (WT) and the rmn mutant livers. Similarly, autophagosomal structures (arrowheads) were observed in both wild-type and rmn mutant livers. Bar, 1 μm. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
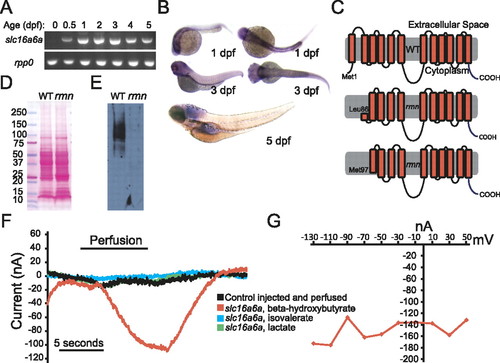
Slc16a6a is a β-hydroxybutyrate transporter. (A) RT–PCR of mature slc16a6a and rpp0 transcripts at the indicated ages. (B) Whole-mount in situ hybridization with slc16a6a riboprobes. (C) The topology of the predicted wild-type and hypothetical rmn Slc16a6a proteins. (D) Ponceau S staining of nitrocellulose membranes following transfer of proteins resolved by sodium dodecyl sulfate polyacrylamide electrophoresis. (E) Immunoblotting with custom anti-Slc16a6a IgGs of the membrane in D. The membrane was deliberately overexposed in order to increase the chance of detecting a smaller protein band in the rmn mutant. (F) Current tracings of Xenopus oocytes expressing Slc16a6a (or control-injected oocytes, in black) that were perfused with monocarboxylic acids (5 mM) for the indicated time period. Oocytes were voltage-clamped to a holding potential of 70 mV. (G) Current–voltage relation of a Slc16a6a-expressing oocyte perfused with β-hydroxybutyrate. |
|
Genetic and nutritional rescue of rmn mutants. (A) Single-cell, homozygous rmn mutant embryo larvae were injected with wild-type zebrafish slc16a6a or human SLC16A6 cDNAs under the control of either bactin2 (broadly expressed) or fabp10a (liver-limited) promoter elements. Larvae were then stained with ORO on 6 dpf. (B) Whole-mount ORO staining of larvae fed from 5 to 12 dpf. The intestinal lumen contents were also stained with ORO, reflecting the lipid content of the larval diet. (C) Whole-mount ORO staining of larvae fed from 5 to 12 dpf and then removed from food. (D) In previously fed rmn mutants, hepatic steatosis appears after 3 d of fasting. (E) Survival curve for animals fed from 5 to 12 dpf and then removed from food (n = 50 larvae of each genotype). The median age at death was 20 ± 2 dpf for wild-type larvae and 18 ± 1.7 dpf for rmn mutant larvae (log rank test: χ2 = 62.3 on one degree of freedom; P = 2.9 × 10-16). PHENOTYPE:
|