- Title
-
Automated reporter quantification in vivo: high-throughput screening method for reporter-based assays in zebrafish
- Authors
- Walker, S.L., Ariga, J., Mathias, J.R., Coothankandaswamy, V., Xie, X., Distel, M., Köster, R.W., Parsons, M.J., Bhalla, K.N., Saxena, M.T., and Mumm, J.S.
- Source
- Full text @ PLoS One
|
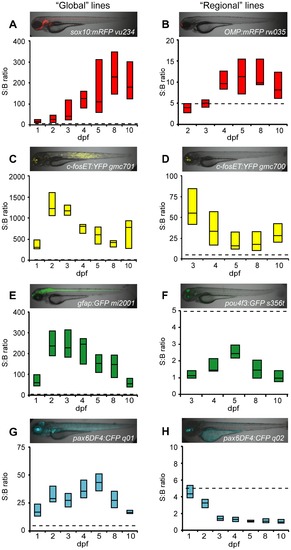
Comparison of fluorescent reporters. Box plots of S:B ratios of transgenic lines expressing different fluorescent reporters across time: A–B) mRFP; C–D) EYFP; E–F) EGFP; G–H) ECFP. Micrographs show the expression patterns of each line. ARQiv scans were performed starting from the earliest day reporter could be detected through 10 dpf. All ‘global’ expressing lines (A,C,E,G) had S:B ratios consistent with excellent HTS assay quality (Z′-factore0.5 across ages tested, see Table 3). In contrast, only “red” fluorophore (B) and YFP (D) expressing “regional” lines produced S:B ratios consistent with HTS (see Table 3). Box plots: bottom line is the 25th percentile, top line the 75th percentile, interface between boxes represents the median (n = 8–24 subjects per line). Note: y-axis ranges differ markedly, dashed line indicates 5:1 ratio of S:B for comparisons of levels across transgenic lines. |
|
ARQiv detection of cell loss and regeneration. Inducible cell-type specific ablation in nitroreductase (NTR) expressing transgenic fish [29], [35] was used to test whether ARQiv could detect the loss and regeneration of fluorescently tagged cells: A–E) Tg(ins:PhiYFP-2A-nfsB, sst2:tagRFP)lmc01 – targeting pancreatic beta cells, and F–J) Double transgenic Tg(pax6-DF4:gap43-CFP)q01; Tg(-3.7rho:YFP-NTR)gmc500 – targeting retinal rod photoreceptors. Beta cell targeting: A) Non-treated control larvae showing PhiYFP-NTR-expressing (i.e., targeted) beta cells (yellow) and neighboring tagRFP-expressing (i.e., non-targeted) delta cells (red) in the pancreas (arrow). B-B*** series) Confocal time-lapse imaging shows reporter expression in beta and delta cells is stable in non-treated control larva from pre-treatment (B and B*, 3 dpf) through post-treatment (B**, 4 dpf) and recovery (B***, 10 dpf). C) MTZ-treated larvae showing loss YFP-NTR-expressing (i.e., targeted) pancreatic beta cells (arrow, absence of yellow). C–D series) Metronidazole (MTZ) treated larvae showing MTZ-induced loss of YFP-NTR-tagged beta cells (absence of yellow; C arrow, and D**, 4 dpf) and subsequent regeneration after MTZ wash out and recovery (D***, 10 dpf). Unperturbed reporter expression in neighboring tagRFP-tagged delta cells (red; C arrow, and D–D***) demonstrates the specificity of the technique. E) ARQiv data demonstrating that beta cell regeneration kinetics can be monitored in late larval to juvenile stage fish (19 to 31 dpf). Paired t-test p-values: • = 0.01, • = 0.33, □ = 0.01. Rod photoreceptor targeting: F) Non-treated control larvae showing YFP-NTR-expressing rod cells (yellow) in eye. G–G***) Confocal time-lapse series showing YFP reporter expression in rod cells (arrowheads) is stable over time in non-treated control larva from pre-treatment (G, 5 dpf) through post-treatment (G*, 7 dpf) and recovery (G***, 10 dpf). H-I series) Metronidazole (MTZ) treated larvae showing MTZ-induced loss of YFP-NTR-tagged rod cells (reduction of yellow in H, and I**, 7 dpf) and subsequent regeneration after MTZ wash out and recovery (I***, 10 dpf). J) ARQiv data demonstrating that rod cell regeneration kinetics can be monitored in larval to fish (5 to 10 dpf); paired t-test p-values: • = 0.001, • = 0.015, □ = 0.001. Data is presented as mean±standard error. All scale bars are 50 μm. |
|
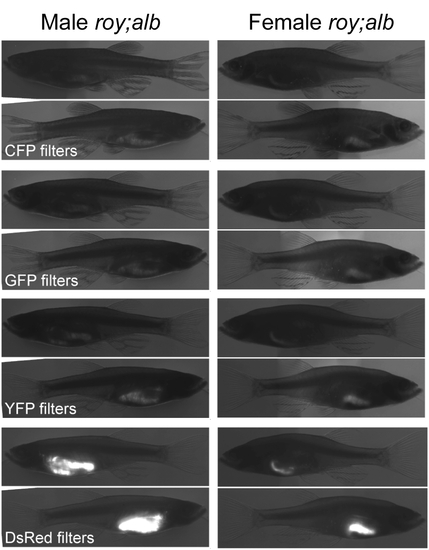
Autoflourescence in adult pigmentation mutants. Autofluorescence profile of roy orbison;albino (roy;alb) double mutant fish at adulthood. Micrographs were taken on an Olympus SZX16 fluorescence steroscope, shown are composites of transmitted and fluorescence images. Autofluorescence was characterized using filter sets for common fluorescent reporters as listed. Region specific autofluorescence is most evident in the gut, and is particularly strong in the red emission wavelengths. |