- Title
-
Lhx2 and lhx9 determine neuronal differentiation and compartition in the caudal forebrain by regulating wnt signaling
- Authors
- Peukert, D., Weber, S., Lumsden, A., and Scholpp, S.
- Source
- Full text @ PLoS Biol.
|
Dynamic expression pattern of lhx2 and lhx9 during regionalization of the caudal forebrain. A double in situ hybridization approach for thalamic development. Embryos were mounted laterally (a, b, c, etc.) or sectioned and the left hemisphere is shown (c2, d2, e2, etc.). Plane of section is indicated in the previous picture with black arrowheads. Asterisks mark the position of the thalamus. Marker genes and stages are indicated (a, b), all other embryos (c–i2) are 48 hpf. lhx2 expression is stained in red and lhx9 is stained in blue. lhx9 expression is revealed in the thalamus at 30 hpf (a). At 42 hpf, lhx9 expression increases and lhx2 expression is detectable ventro-posteriorly within the lhx9 domain (b). At 48 hpf, lhx2 and lhx9 overlap in the Th (c) and cross-section analysis reveals an overlap of both markers within the mantle zone of the thalamus (c2). The shh-positive mid-diencephalic organizer (MDO) is located anterior to the lhx9 positive thalamus (d), and a cryo-section reveals a gap between both expression domains (d2). Helt expression in the rostral thalamus (rTh) and pretectum (PTec) abuts the lhx9 expression (e, e2). neurog1 marks the thalamic territory (f) and cross-section in (f2) shows that neurog1 marks the subventricular zone (SVZ; white bar) and does not overlap with the expression domain of lhx9 in the mantle zone. The thalamus expression domain of lhx9 overlaps with the pattern of id2a in the medial part of the mantle zone (g, g2, black bar). lef1 as a marker of post-mitotic thalamic neurons shows co-expression with lhx9 in the MZ (i, i2 black bar). Notably, lhx9 expression is seen also in the epiphysis (Ep). The thalamic lhx9 expression domain abuts the wnt3a expression domain in the epithalamus (ETh, g, g2). ETh, epithalamus; HyTh, hypothalamus; Mtz; marginal tecal zone; pTu, posterior tuberculum; Tec, tectum; Tel, telencephalon. |
|
Differentiation of thalamic neurons is stalled in lhx2/lhx9 morphant embryos. Analysis of embryos for neuronal differentiation in double morphant embryos at 48 hpf, lateral view (a, b, c, etc.), and cross-section of left hemispheres (a2, b2, c2 etc.) of the same embryo are shown. The expression domain of the neuronal precursor deltaA at the ventricular zone (VZ) is vigorously expanded in double morphant embryos (a–b2). Expression of the progenitor marker neurog1 marking the subventricular zone (SVZ, white bars) is also broadened in lhx2/lhx9 morphant embryos compared to control embryos (c–d2). However, the thalamus-specific terminal differentiation markers, id2a and lef1, are down-regulated in the mantle zone of the cTh (MZ, black bars) of Lhx2/Lhx9-deficient embryos (e–h2). The postmitotic marker gbx2 shows no alteration in the compound morphant embryos (i–j2). The number of cells expressing the pan-postmitotic neuronal marker elavl3 is strongly decreased in the double morphant embryos shown by a confocal microscope section of an transgenic Elavl3:GFP transgenic embryos (k, l). The deltaA and neurog1 positive precursor pool in the ventricular/subventricular zone (blue and green domain) expands on the expense of the post-mitotic thalamic neurons (red domain) in the mantle zone in lhx2/lhx9 morphant embryos (m, n). ETh, epithalamus; HyTh, hypothalamus; MDO, mid-diencephalic organizer; MZ, mantle zone; pTu, posterior tuberculum; VZ, ventricular zone. |
|
Lhx2 promotes thalamic neurogenesis. At 24 hpf, DNA (indicated in red) was injected into the brain ventricle followed by electroporation approach (a). To validate the specificity and efficiency, we targeted one hemisphere of the thalamus territory with EGFP DNA at 24 hpf. We find a co-localization with the thalamus-specific marker barhl2:mCherry at 48 hpf (b). Analysis of cross-sections reveal that electroporation of EGFP DNA does not alter the expression of lef1 in wt embryos (c). Furthermore, we find strong down-regulation of lef1 in lhx2/lhx9 morphant embryos, which is not altered by EGFP DNA electroporation (d). After electroporation of lhx2 DNA, we observe an unaltered expression of id2a, lef1, and Elavl3-GFP expression within the endogenous expression site in the electroporated hemispheres (e, g, i). Electroporated side was identified by an ISH against lhx2 mRNA in red. However, electroporation of lhx2 DNA at 24 hpf can restore the expression of id2a, lef1, and Elavl3-GFP in Lhx2/Lhx9-deficient embryos (f, g, j; asterisk). Notably, electroporation of Lhx2 can ectopically induce id2a expression in the basal plate—that is, in the pTu (f). pTu, posterior tuberculum; RP, roof plate, Tec, tectum; Tel, telencephalon. |
|
Knock-down of Lhx2/Lhx9 leads to an expansion of the Wnt positive epithalamus. A lateral view (a, b, c, etc.) and a cross-section (a2, b2, c2, etc.) of the left hemisphere of the same embryo at 48 hpf are displayed. Thalamus is marked by asterisks. Section plane of the cross-section is indicated by black arrowheads. In control MO injected embryos, lmx1b.1 expression domain marks the MDO and the dorsal RP (a, a2). Knock-down of Lhx2/Lhx9 leads to an expansion of both areas into the thalamic territory (b, b2). wnt3a marks the epiphysis but not the habenula territory (c, c2). In Lhx2/Lhx9-deficient embryos, wnt3a expression is ectopically activated in the dorsal part of the thalamus (d, d2). Subsequently the expression of Wnt target genes such as axin2 (e, e2) as well as the Wnt reporter line 7×TCF-Xla Siam:GFP ia4 (g, g2) shows an expanded expression domain in compound morphant embryos (f, f2 and h, h2). Ep, epiphysis; Hb, habenula; pTu, posterior tuberculum. |
|
Expression and regulation of protocadherin10b in the thalamus. Lateral views and corresponding cross-sections of the left hemisphere of the same embryo at 48 hpf are displayed. Exceptions are a horizontal section in (c2) and dorsal view in (d). Asterisks mark the thalamic territory. pcdh10b expression abuts the expression domain of lhx9 in the mantle zone (MZ, black bar; a, a2). The roof plate marker, wnt3a, is adjacently expressed to the pcdh10b expression in the thalamus (b, b2). Expression of pcdh10b in the thalamus abuts posteriorly the expression domain of gsx1 and therefore respects the border to the pretecum (c) shown in a dorsal view (c2). Overexpression of lhx2 DNA via electroporation leads to a unilateral downregulation of pcdh10b expression (dorsal view, d; d2). Control embryos show pcdh10b expression in the cTh (d, d2). In lhx2 mutant embryo knocked-down for lhx9, pcdh10b expression expands into the pretectum (e), and the ventricular expression expands into the MZ (e2, white bar). Treatment of embryos with the Wnt signaling agonist BIO from 16 hpf to 48 hpf leads to an expansion of pchd10b expression into the pretectum (f, white arrow), however the expanded VZ is not detectable (f2, white bar). Although the gross morphology is altered, pcdh10b expression shows similar broadening in axin1 mutant embryos (g, g2). Consequently, blocking of Wnt signaling by IWR-1 treatment from 16 hpf to 48 hpf leads to a severe downregulation of pcdh10b (h, h2). Embryos with ubiquitous expression of the Wnt inhibitor Dkk1 after heat shock activation at 10 hpf leads to a downregulation of pcdh10 expression at 48 hpf (i, i2). Knock-down of Wnt3a in the Lhx2/Lhx9-double-deficient embryos leads to a rescue of the expansion into the pretectum (j), however the lateral expansion of the VZ is still detectable (j2). Canonical Wnt signaling—that is, Wnt3a—is required for induction of pcdh10b expression in the thalamic ventricular zone, whereas Lhx2/Lhx9 inhibits pcdh10b expression in the mantle zone of the cTh (k). |
|
Protocadherin10b is required to maintain integrity of thalamus. Dorsal views of the left hemisphere of embryo at 42 hpf (a–c), 48 hpf (d–i), and 3 dpf (j–l) are displayed. To visualize orientation of the figures, small sketches accompany the experiments showing the thalamus (Th) in dark grey and the rostral thalamus (rTh)/pretectum (PTec) in light grey. At 4 dpf, the anatomy of the caudal forebrain is visualized by a confocal microscopy analysis of ubiquitous nuclei staining by Sytox green (m–o). At 42 hpf, gbx2:GFP expression marks the thalamus as well as the position of the diencephalic-mesencephalic border (DMB) by the position of the posterior commissure (PC). Knock-down of Lhx2/Lhx9 leads to the appearance of gbx2:GFP positive cells posterior to endogenous expression domain (b, white arrow). In embryos knocked down for Pcdh10b, thalamic gbx2:GFP cells appear similarly to (b) in the pretectum (c, white arrows). Analysis of lhx2/lhx9 morphant embryos and pcdh10b morphant embryos by a double ISH approach for lhx9/gsx1 (d–f). lhx9 marks the thalamus and gsx1 the pretectum seen in a dorsal view (d). In Lhx2/Lhx9 morphant embryos, the expression pattern of lhx9 and gsx1 intermingles (e, white arrow) similar to the phenotype observed in pcdh10b morphant embryos (f; white arrows). Confocal sectioning of lhx2/lhx9 double morphant embryos in vivo reveals mixing between Tal1:GFP positive and the neurog1:RFP positive cells in the cTh (g–h2, white arrows). A similar intermingling phenotype is detectable in pcdh10b morphant embryos at 48 hpf (i, i2). At 3 dpf, the rTh is marked by gad1 by a fluorescent ISH (j, j2). After knock-down of Lhx2/Lhx9, gad1 positive cells can be found in the territory of the cTh (k, white arrows); furthermore, in Pcdh10b-deficient embryos, gad1 positive can also be found in the cTh (l, l2). Lateral views of the caudal forebrain show three cell nuclei loose border zones: the border between prethalamus and thalamus, the ZLI (white dashed lines), the one between the thalamus and the pretectum (red arrows), and the one between pretectum and midbrain DMB (white dashed lines). The border zone between the thalamus and the pretectum is not detectable in lhx2/lhx9 morphant embryos (n). Similarly, this demarcation is also missing in pcdh10b morphant embryos (o), whereas the ZLI and the DMB are not affected. Tec, tectum; Teg, tegmentum. EXPRESSION / LABELING:
|
|
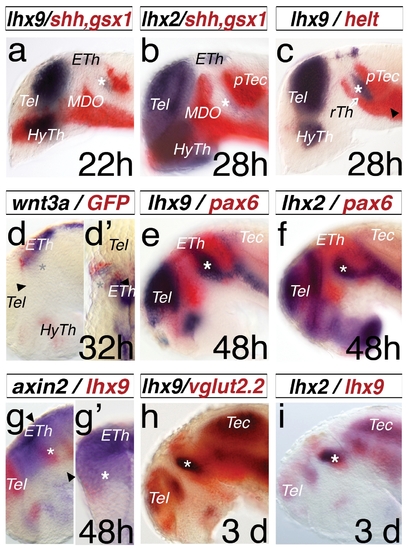
Expression pattern of lhx2 and lhx9 during thalamus development. A double in situ hybridization approach was used for analysis. All embryos were mounted laterally with stages indicated, except (d2) is a dorsal view and (g2) is a cross-section of the left hemisphere. lhx9 reveals an onset of expression in the thalamus (Th) at 22 hpf (a, asterisk), limited anteriorly by shh, a marker of the MDO and posteriorly by gsx1, a marker of the pretectum (PTec). At 28 hpf, lhx2 shows an onset of expression in the thalamus (b, asterisk). Within the thalamus, at 28 hpf helt marks the rostral thalamus (rTh) and the pretectum (c), however the lhx9 expression domain shows no overlap with the helt domain. The epithalamus is marked by the Wnt ligand, wnt3a, and the expression of the Wnt reporter 7×TCF-siam:GFP (d). The dorsal view reveals lateral a stronger expression of gfp-mRNA in comparison to the wnt3a pattern (d2). At 48 hpf, lhx2 and lhx9 show specific expression patterns in the telencephalon (Tel), thalamus (asterisk), and ventral to the tectum (Tec), indicated by the overlapping expression domain of pax6a, marking the alar plate of the forebrain during development (e, f). axin2 expression in the thalamus co-localizes with the lhx9 expression. (g, g2). vglut2.2, a marker of glutamatergic neurons in the relay thalamus (cTh), shows an overlapping expression domain with lhx9 (h). Both genes, lhx2 and lhx9, mark the thalamus at 3 dpf (i). ETh, epithalamus; HyTh, hypothalamus; MDO, mid-diencephalic-organizer; PTec, pretectum; RP, roof plate; rTh, rostral thalamus; Tec, tectum ; Tel, telencephalon. |
|
Efficient knock-down of lhx2 and lhx9 during forebrain development. To validate the efficiency of the lhx2 and lhx9 splice-site Morpholino-antisense oligomere approach, we isolated cDNA from injected and non-injected embryos at 48 hpf. A PCR approach, with primers flanking exon1 and exon2 of lhx2, demonstrates a suppression of the splicing event of intron1 (1.5 kb) in five individual embryos injected with lhx2 MO (emb1–5) compared to a control embryo (con, 221 bp) (a). A similar effect is demonstrated in injected lhx9 MO embryos 1–4 (emb1–4; b), which display a non-splicing event of intron1 (993 bp), compared to control embryos (con, 231 bp) (b). An antibody against acetylated tubulin shows midline crossing axons anterior (AC, anterior commissure) and posterior (POC, post-optic commissure) in the telencephalon (c). In lhx2/lhx9 double morphant embryos, both commissures do not cross the midline (arrow, d). Single in situ hybridizations of embryos at 48 hpf are displayed by a lateral view (e–l). Knock-down of Lhx2 and Lhx9 leads to a decrease of sema3d expression in postoptic commissure (POC, arrow; e, f). The morphant analysis of single knock-down, either lhx2 or lhx9, shows that lef1 expression is unaltered in the thalamus (h, j), compared to the control embryos (g, i, k). In the lhx2 mutant embryos, lef1 expression in the thalamus shows a weak alteration (l). HyTh, hypothalamus; MDO, mid-diencephalic-organizer; pTu, posterior tuberculum; RP, roof plate; Tec, tectum; Tel, telencephalon. |
|
lhx2/lhx9 morphant embryos show defect in thalamic neuron differentiation. A single in situ hybridization approach was used for analysis and all embryos were mounted laterally except in (I2, j2) showing cross-section of left hemispheres. Stages are indicated. In Lhx2/Lhx9-deficient embryos, lef1 expression in the thalamus (asterisk) is unaltered at 24 hpf but down-regulated at 3 dpf (a–d). Similarly, the Wnt target gene axin2 shows no alteration in Lhx2/Lhx9-deficient embryos at 20 hpf (e, f), however at 3 dfp an up-regulation can be detected in the mid-diencephalon (g, h). In control MO embryos, wnt1 is expressed at 48 hpf in the roof plate (RP) and lhx2/lhx9 morphant embryos display an expansion of the wnt1 expression domain into the thalamic territory (i–j2). In contrast tcf7l2 shows no alteration in the expression pattern at the same stage in the caudal forebrain. HyTh, hypothalamus; pTu, posterior tuberculum; RP, roof plate; Tec, tectum; Tel, telencephalon. |
|
The thalamic expression of protocadherin10b and its regulation. All embryos are analyzed by a single in situ hybridization approach and mounted laterally, with stages indicated, except (c2) shows a cross-section and the left hemisphere is displayed. In the thalamus (asterisk) pcdh10b reveals an onset of expression in segmentation phase (18 hpf), which increases during development (a, b). Knock-down of Lhx2/Lhx9 leads to an expansion of pcdh10b expression into the pretectum (pTec, c), as well as of the ventricular zone (VZ, white bar, c2). Black arrowheads indicate the plane of a cross-section. To validate the efficiency of pharmacological treatment with the Wnt signaling agonist BIO or antagonist IWR-1, we also analyzed under the same conditions the Wnt target gene axin2. Treatment with the Wnt signaling agonist BIO demonstrates an up-regulation of axin2, displayed lateral (d, e). Axin2 expression is upregulated in axin1 mutant embryo masterblind (mbl, f). The treatment of embryos with the Wnt signaling antagonist IWR-1 leads to a loss of axin2 in the diencephalon (g, h). We find a similar reduction of axin2 expression in embryos expressing Dkk1 post-heat-shock at 16 h (i). Treatment of embryos with the Wnt agonist BIO has no effect in the expression of shh or pax6a in the forebrain (j–m). In contrast, embryos treated with the antagonist IWR-1 after endogenous pcdh10b induction between 24 hpf and 48 hpf show no change in pcdh10b expression pattern. HyTh, hypothalamus; pTec, pretectum; pTu, posterior tuberculum; RP, roof plate; Tec, tectum; Tel, telencephalon. |
|
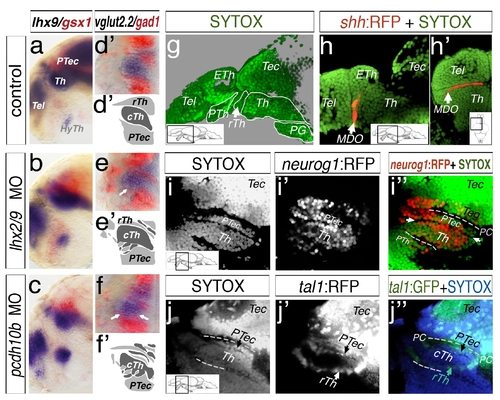
Mapping of the diencephalon in larval stage via SYTOX nuclei staining. Analyses at 48 hpf, lateral view (a, b, c) and dorsal sections of left hemispheres (d–f2) are shown. Lhx9 marks the thalamus (a) and gsx1 the pretectum (a). In lhx2/lhx9 and pcdh10b morphant embryos, the expression domains overlap. A similar intermingling of expression domains is visible in embryos stained for vglut2.2 and gad1 (d–f2). Embryos have been analyzed at 4 dpf by a confocal microscopy analysis of ubiquitous nuclei staining by Sytox (g–j3). The analyzed section of the lateral view except dorsal view (h2) is indicated by a schematic drawing (insert). A sytox staining in green reveals structures of the forebrain and midbrain (g). To confirm the position of the thalamus, we analyzed the shh:RFP transgenic line, marking the MDO anterior to the thalamus (Th, b, b2). The position of the thalamus and pretectum (PTec) was mapped in the neurog1:RFP transgenic line (i–i3). To distinguish between the caudal thalamus (cTh) and pretectum, we also analyzed the tal1:GFP transgenic line. It labels GABAergic neurons of the rostral thalamus (rTh) and pretectum and therefore identifies the cell nuclei loose border zone between thalamus and pretectum (j–j3). ETh, epithalamus; HyTh, hypothalamus; MDO, mid-diencephalic-organizer; PC, posterior commissure; PG, preglomerular complex; PTec, pretectum; PTh, prethalamus; pTu, posterior tuberculum; RP, roof plate; rTh, rostral thalamus; Tec, tectum; Tel, telencephalon; Th, thalamus. |