- Title
-
An Intermediate Level of BMP Signaling Directly Specifies Cranial Neural Crest Progenitor Cells in Zebrafish
- Authors
- Schumacher, J.A., Hashiguchi, M., Nguyen, V.H., and Mullins, M.C.
- Source
- Full text @ PLoS One
|
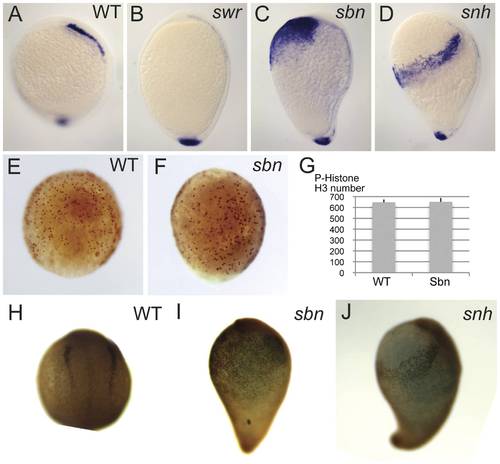
Reduction of BMP signaling in BMP pathway mutants affects the number of NCPC specified. foxd3 expression at bud stage in wild-type (A), swr (B), sbn (C), and snhty68a (D). P-histone H3 expression at 80% epiboly stage in wild-type (E) and sbn (F). (G) Quantification of the number of P-histone H3 positive cells in wild-type (n = 3) and sbn (n = 3). Foxd3 protein expression at the 2-somite stage in wild-type (H), sbn (I), and snhty68a (J). |
|
Reduction of BMP signaling in wild-type embryos causes expansion or loss of NCPC in dosage-sensitive manner. foxd3 expression in chd mRNA injected embryos (A) and smad5 MO injected embryos (B) at the end of gastrulation. (A) Injection of a low dose of chordin mRNA (50 pg) generates weaker NCPC phenotypes (WT = normal or very mild expansion, “snh” = moderate expansion), whereas a high dose (200 pg) leads to strong phenotypes (“sbn” = large expansion, or “swr” = loss). (B) Injection of a low 2.5 ng dose of smad5 MO leads to “snh” and “sbn” phenotypes. Injection of a high 4 ng dose of smad5 MO leads to “sbn” and “swr” phenotypes exclusively. |
|
NCPC domains when BMP signaling is reduced in somitabun and snailhouse and increased in swirl embryos. foxd3 expression at the end of gastrulation in tBR mRNA injected embryos of sbn (A) and of snh (B), and smad5 mRNA injected into swr embryos (C). (A) Injection of tBR mRNA into sbn mutants leads to the majority of embryos displaying a “swr” phenotype. (B) Injection of a low 15 pg dose of tBR mRNA into snh mutants leads to nearly equal numbers of “snh” and “sbn” phenotypes. Injection of a higher 100 pg dose leads to the majority of embryos displaying the “sbn” phenotype and also a percentage displaying a stronger “swr” phenotype. (C) Injection of a low 30 pg dose of murine smad5 mRNA results in nearly half of embryos displaying a “sbn” phenotype. Injection of a higher 150 pg dose results in a small percentage of embryos displaying the “swr” phenotype, and the rest of the embryos divided between “sbn”, “snh”, and WT phenotypes. |
|
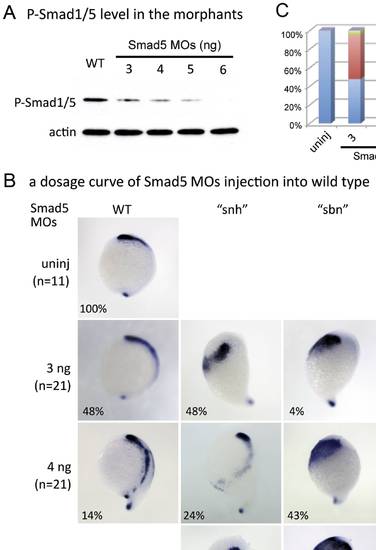
Levels of P-Smad1/5 correlate with the strength of NCPC phenotype in smad5 morphant embryos. (A) P-Smad1/5 levels in smad5 morphant embryos. Embryos injected with increasing higher concentrations of smad5 MOs show decreasing P-Smad1/5 levels relative to uninjected controls. Actin was used as a loading control. (B, C) Expression of foxd3 in embryos injected with increasing doses of smad5 MOs. Injection of a low dose of smad5 MOs (3 ng) leads to WT, “snh” and “sbn” phenotypes, whereas injection of higher doses of smad5 MOs (5 ng, 6 ng) lead to “sbn” and “swr” phenotypes. The embryos used for in situ hybridization of foxd3 in Fig. 4B are from the same batch of injected embryos used for Western blotting in Fig. 4A. |
|
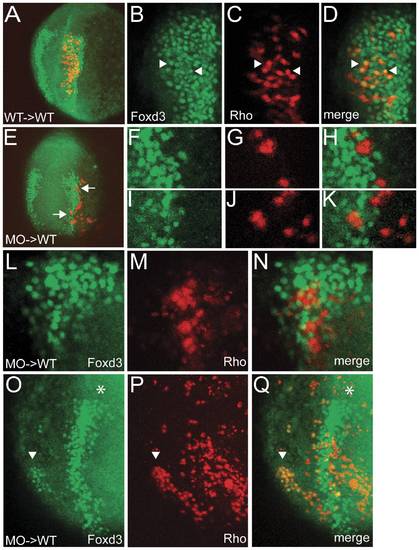
BMP signaling is required cell autonomously in NCPCs for their specification. (A) Z-projection of confocal sections showing that wild-type donor cells transplanted into wild-type hosts readily express the neural crest marker Foxd3 at the 3-somite stage. (B,C,D) Single confocal section of the embryo in A. Foxd3 (B) and lineage tracer rhodamine dextran (C) are found in the same cells (D, arrowheads). (E) Z-projection of confocal sections showing that smad5 morphant donor cells within the neural crest region of a wild-type host do not express the neural crest marker Foxd3. (F, G, H, I, J, K) Single confocal sections of the cells indicated by arrows in E. Foxd3 (F, I) and lineage tracer (G, J) do not colocalize (H, K). (L, M, N) Single confocal section of a different host embryo containing smad5 morphant cells (Rho) within the neural crest region that do not express Foxd3. (O, P, Q) Z-projection of confocal sections of chimera in which donor cells were induced as ectopic neural crest. Foxd3 (O) and lineage tracer (P) colocalize (Q) in a patch of cells (arrowhead) located ventrally from the normal neural crest domain (asterisk). |