- Title
-
Neuronal Neuregulin 1 type III directs Schwann cell migration
- Authors
- Perlin, J.R., Lush, M.E., Stephens, W.Z., Piotrowski, T., and Talbot, W.S.
- Source
- Full text @ Development
|
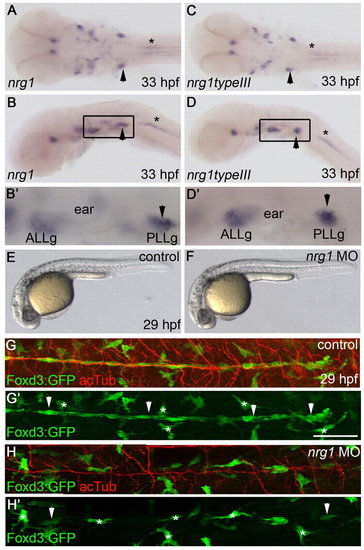
Neuregulin 1 is required for Schwann cell migration. (A-D2) nrg1 (A,B,B2) and nrg1 type III (C,D,D2) mRNA expression at 33 hpf, shown in dorsal (A,C) and lateral (B,D) views. (B2,D2) Higher magnification of the boxed regions in B,D. Arrowheads mark the PLLg and asterisks mark motoneurons. (E,F) Lateral views of control-injected (E) and nrg1 morpholino-injected (F) zebrafish embryos at 29 hpf. (G-H2) Lateral views of control-injected (G,G2) and morphant (H,H2) embryos carrying the Foxd3:GFP transgene (green) and stained with anti-acetylated tubulin (acTub, red) at 29 hpf. Schwann cells are marked by arrowheads and stellate-shaped pigment cells by asterisks. In lateral views, anterior is to the left and dorsal is up. In dorsal views, anterior is to the left. ALLg, anterior lateral line ganglion; PLLg, posterior lateral line ganglion. Scale bar: 50 μm. |
|
nrg1z26 specifically disrupts the Nrg1 type III isoform. (A) Protein structure of zebrafish Nrg1 type III. The z26 lesion in the cysteine-rich domain (CRD) of the type III-specific domain (dark blue) changes a conserved cysteine (C) at position 95 to an arginine (R). TM, transmembrane domain. Scale bar: 40 amino acids. (B) Comparison of zebrafish and human Nrg1 type III CRD sequences. The conserved cysteine mutated by the z26 lesion is shaded in blue. (C,D) Sequence traces from wild-type and z26 mutant zebrafish larvae illustrating the T-to-C lesion. (E,F) Lateral views of 5 dpf wild-type and nrg1z26 mutant sibling embryos. (G,H) Lateral views of 30 hpf nrg1z26/+ siblings (G) and nrg1z26 mutants (H) stained with anti-acetylated tubulin (acTub, red). Arrowheads mark the PLLg, asterisks mark pigment cells. Embryos were genotyped after imaging. (I,J) PLLg of a 53 hpf nrg1z26/+ sibling (I) and nrg1z26 mutant (J) stained with anti-Sox10 (red) and anti-acTub (blue). Asterisks mark Schwann cells associated with the ganglion. (K,L) PLLg of 26 hpf nrg1z26/+ sibling (K) and nrg1z26 mutant (L) stained with anti-phospho-Histone H3 (pH3) (red) and anti-acTub (blue). Arrowhead marks Foxd3:GFP+ cells that are co-labeled with pH3. (G-L) Schwann cells are marked by Foxd3:GFP (green, arrows). Anterior is to left, dorsal is up. (I-L) Embryos were genotyped before imaging and images are a projection of either three (I,J) or four (K,L) 3 μm sections taken at the middle of each PLLg. Scale bars: 50 μm in G,H; 25 μm in I-L. |
|
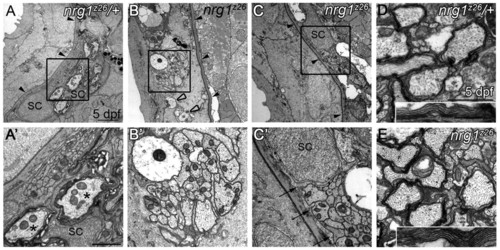
Nrg1 type III is required for myelin formation in the peripheral but not central nervous system. (A-C2) Transmission electron microscopy (TEM) images of transverse sections through the posterior lateral line nerve (PLLn) of zebrafish larvae of the indicated genotypes. (A,A2) At 5 dpf, nrg1z26/+ nerves are beneath the epidermal basement membrane (black arrowheads) and Schwann cells (SC) have begun to myelinate axons (asterisks). (B,B2) nrg1z26 mutant nerves that lack Schwann cells fail to transition across the basement membrane (black arrowheads) and remain in the epidermis where they are unmyelinated and defasciculated (open arrowheads). (C,C2) nrg1z26 mutant nerves with ‘escaper’ Schwann cells are correctly positioned beneath the epidermal basement membrane (black arrowheads), but Schwann cell processes (arrows) fail to myelinate axons within the bundle. (A2,B2,C2) Higher magnification of the boxed regions in A,B,C. (D,E) TEM of transverse sections through the ventral spinal cord of nrg1z26/+ (D) and nrg1z26 mutant (E) siblings. Insets show myelin around the Mauthner axon. All images were taken at the level of the fifth somite. Scale bars: 2 μm in A,B,C; 1 μm in A2,B2 0.5 μm in C2 1 μm in D,E; 0.5 μm in D,E insets. |
|
Nrg1 type III is required in neurons and ErbB receptors are required in all Schwann cells. (A) Wild-type cells marked with a Texas Red-Dextran lineage tracer were transplanted into nrg1z26 mutant hosts carrying the Foxd3:GFP (green) transgene at the blastula stage. (B-B2) Lateral view of genetic chimera generated by transplanting wild-type cells (red) into the nrg1z26 mutant. Arrowheads point to nrg1z26 mutant Schwann cells, which have migrated along axons (arrows) of wild-type neurons (asterisks). Open arrowhead marks a wild-type Schwann cell. (C) Wild-type cells carrying the Foxd3:GFP transgene (green) labeled with a Texas Red-Dextran lineage tracer were transplanted into erbbst50 mutant hosts also carrying the Foxd3:GFP transgene at the blastula stage. (D-D2) Lateral view of genetic chimera generated by transplanting wild-type cells (red) into the erbbst50 mutant. All Schwann cells (arrowheads) are labeled by the wild-type lineage tracer. Anterior is to the left and dorsal is up in all images; embryos were genotyped after imaging. Scale bars: 50 μm. |
|
Overexpression of human NRG1 type III in all neurons leads to expression of Schwann cell markers in the spinal cord. (A-J) Lateral views of human NRG1 (hNRG1) type III-overexpressing transgenic zebrafish embryos (B,D,F,H,J) and control siblings (A,C,E,G,I) stained by in situ hybridization for the indicated genes. Anterior is to the left and dorsal is up. Embryos were genotyped before imaging. Transgenic embryos have expanded foxd3 (C,D), mbp (E,F) and krox20 (I,J) expression in dorsal hindbrain (brackets) and spinal cord (arrowheads). olig2 is only slightly expanded in hNRG1 type III-overexpressing embryos (G,H). (K-M) Cryosections of embryos showing krox20 expression. krox20-expressing cells are present within the neural tube (black arrowheads) and along nearby nerves (asterisks) of hNRG1 type III-overexpressing embryos (L,M), but not in control embryos (K). The PLLn is marked by open arrowheads. EXPRESSION / LABELING:
PHENOTYPE:
|
|
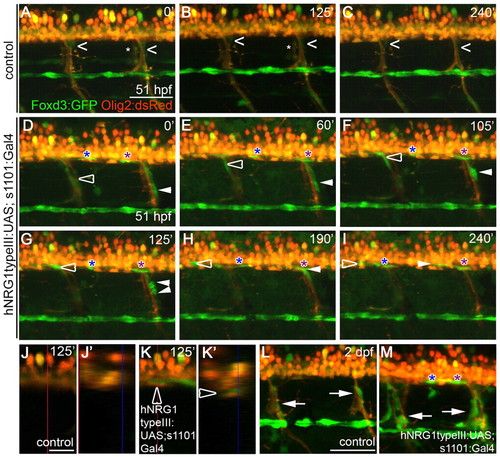
Overexpression of hNRG1 type III in all neurons leads to the ectopic migration of Schwann cells into the spinal cord. (A-K2) Images from in vivo time-lapse movies. Schwann cells are marked by the Foxd3:GFP transgene (green) and oligodendrocytes and motoneurons are marked by Olig2:dsRed (red). Numbers at top right denote time (minutes) elapsed since the start of the movie. (A-C) Schwann cells (carets) along motor nerves undergo little movement in the control embryo; asterisks mark motor nerves on the contralateral side. (D-I) Embryos overexpressing hNRG1 type III in all neurons. The open arrowhead follows a Schwann cell that migrates up a motor nerve and into the spinal cord; the white arrowhead follows a Schwann cell that divides and produces one daughter that moves into the spinal cord; and blue and purple asterisks follow Schwann cells already present in the spinal cord as they migrate along spinal axons. (J,K) Lateral views of single scans from in vivo time-lapse movies of control (J) and transgenic (K) embryos at 125 minutes. (J2,K2) Orthogonal view of the yz plane indicated by the vertical red lines in J and K. The open arrowhead marks the Schwann cell that is tracked by the open arrowhead in D-I. (L,M) Excess Schwann cells (arrows) are present along motor nerves of embryos overexpressing hNRG1 type III (M) as compared with control embryo (L) at 2 dpf. Anterior is to the left, dorsal is up. Comparable regions over the yolk extension were imaged. Embryos were genotyped after imaging. Scale bars: 50 μm in A-I,L-M; 20 μm in J,K. |
|
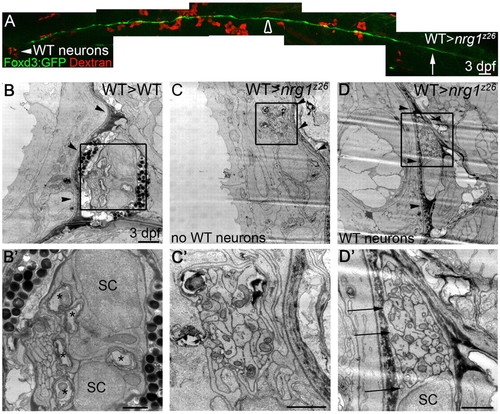
Schwann cells can migrate along nrg1z26 mutant axons in the presence of wild-type neurons. (A) Lateral view of 3 dpf genetic chimera generated by transplanting Dextran-labeled wild-type cells (red) into an nrg1z26 mutant with Foxd3:GFP-labeled Schwann cells (green). White arrowhead marks wild-type neurons in the ganglion; arrow marks the most posterior Schwann cell; open arrowhead marks the region that was examined by TEM in C,D. Anterior to left, dorsal is up; embryo genotyped after imaging. Scale bar is 50 μm. (B-D2) TEM of transverse sections of the PLLn of larvae of the indicated genotypes at somite 10. (B,B2) The PLLn of the wild-type chimera is in its mature location beneath the basement membrane (arrowheads), and Schwann cells (SC) have begun to myelinate axons (asterisks). (C,C2) The contralateral nerve of the rescued nrg1z26 mutant chimera lacks wild-type neurons, has no visible Schwann cell nuclei or processes, and fails to properly transition across the basement membrane (arrowheads). (D,D2) The PLLn of nrg1z26 mutant chimera with wild-type neurons successfully transitioned across the epidermal basement membrane (arrowheads), and Schwann cell processes (arrows) envelop all axons in the bundle. (B2,C2,D2) Higher magnifications of boxed regions in B,C,D. Scale bars: 50 μm in A; 2 μm in B,C,D; 1 μm in B2,C2,D2. |
|
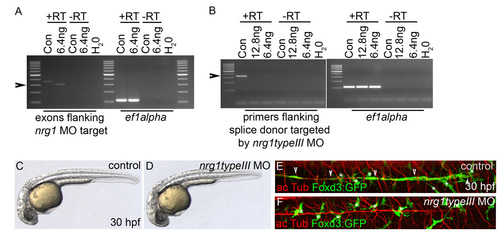
nrg1 type III is required for Schwann cell migration. (A) Reverse transcriptase PCR (RT-PCR) of 29 hpf embryos injected with buffer (control) or with 6.4 ng nrg1 morpholino (MO). Primers used were to ef1a or to exons flanking the nrg1 MO target, with or without reverse transcriptase. The nrg1 transcript is improperly spliced in 29 hpf embryos injected with 6.4 ng nrg1 morpholino as compared with control-injected embryos (arrowhead). (B) RT-PCR of 30 hpf embryos injected with buffer (control), 12.8 ng or 6.4 ng nrg1 type III morpholino. Primers were designed to ef1a or to regions flanking the splice donor targeted by nrg1 type III morpholino, with or without reverse transcriptase. nrg1 type III transcript is reduced in 30 hpf embryos injected with 6.4 ng nrg1 type III morpholino and absent upon 12.8 ng injection as compared with control-injected embryos (arrowhead). A 3.4 kb PCR product is expected if the intron is retained due to aberrant splicing, which would not be amplified under these PCR conditions. Further studies were performed on embryos injected with 6.4 ng of morpholino. Con, control-injected; H2O, water control; +RT, with reverse transcriptase; -RT, same samples without reverse transcriptase. Primers to ef1a were used as a control. (C,D) Lateral views of control-injected embryos (C) and embryos injected with 6.4 ng nrg1 type III morpholino (D) at 30 hpf. (E,F) Lateral views of 30 hpf control- and morphant-injected embryos carrying the Foxd3:GFP transgene (green) and stained with anti-acetylated tubulin (acTub, red). Schwann cells have migrated along PLL axons in control (E) but not morphant (F) embryos. Asterisks mark pigment cells, which are also Foxd3:GFP+. (C-F) Anterior is to the left and dorsal to the top. |
|
Early and late Schwann cell markers are absent in nrg1z26 mutants. (A,B) Dorsal views of nrg1 type III mRNA expression in nrg1z26/+ (A) and nrg1z26 (n=3/3) embryos at 35 hpf. Arrow marks PLLg. (C,D) sox10 marks Schwann cells along the PLLn (arrowheads) in a wild-type embryo (C), but is almost absent along the PLLn of an nrg1z26 mutant (n=11/11) (D). (E-H) Myelin basic protein mRNA (E) and protein (G) is expressed along the PLLn (arrowheads) of wild-type embryos, but not in nrg1z26 mutants (F, n=3/3; H, n=6/6). Scale bar: 50 μm. PHENOTYPE:
|
|
Schwann cells migrate within the spinal cord of embryos overexpressing human NRG1 type III. (A,B) Lateral views of single scans from in vivo time-lapse movies of a control embryo (A; see Movie 1 in the supplementary material) and an embryo overexpressing human NRG1 type III in all neurons (B; see Movie 2 in the supplementary material) at 125 minutes. Schwann cells are marked by the Foxd3:GFP transgene (green) and oligodendrocytes and motoneurons are marked by Olig2:dsRed (red). (A2,B2) Orthogonal view of the yz plane at the location of the vertical red lines in A and B. Arrowhead marks PLLn (A2,B2); arrow points to Foxd3:GFP staining within the Olig2:dsRed-marked spinal cord (B2). The open arrowhead marks the Schwann cell that is tracked by the open arrowhead in Fig. 6D-I,K,K2. Anterior is to the left, dorsal is up. Comparable regions over the yolk extension were imaged. Embryos were genotyped after imaging. Scale bar: 50 μm. |

Unillustrated author statements PHENOTYPE:
|