- Title
-
Motoneurons are essential for vascular pathfinding
- Authors
- Lim, A.H., Suli, A., Yaniv, K., Weinstein, B., Li, D.Y., and Chien, C.B.
- Source
- Full text @ Development
|
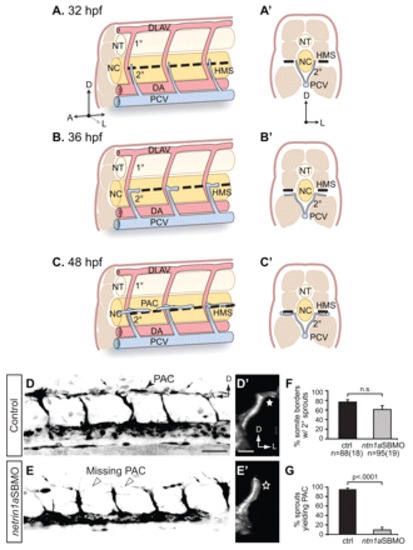
netrin 1a is required for turning of secondary sprouts at the horizontal myoseptum. (A-C2) Lateral (A-C) and corresponding transverse views (A2-C2) of parachordal chain (PAC) formation, showing dorsal aorta (DA, red), posterior cardinal vein (PCV, blue), horizontal myoseptum (HMS, dashed line), primary intersomitic vessels (1°, red) and secondary sprouts (2°, blue). DA and primary sprouts omitted for clarity. (A,A2) Secondary sprouts emerge from the PCV at <32 hpf and grow dorsally to the HMS. (B,B2) Dorsal growth stops at the HMS and sprouts turn and grow laterally. (C2) Lateral growth stops at the lateral-most aspect of the muscle, and the sprout then turns anteriorly and posteriorly to form the PAC. (D-E2) Removing netrin 1a function in 48 hpf plcg1y10; fli1a:egfpy1 zebrafish embryos. (D,E) Lateral views, reversed contrast. (D2,E2) Transverse view volume renderings of single hemisegments from D,E. (D,D2) In uninjected controls, secondary sprouts grow from the PCV and turn at the HMS (star, D2) to form the PAC (arrowhead, D). The fli1a:egfpy1 transgene also labels some dimly fluorescent non-endothelial cells near the HMS (small arrows, D,E). (E,E2) In netrin 1a morphants, secondary sprouts usually fail to turn laterally (star, E2) and form the PAC (arrowheads, E). (F,G) Secondary sprouts were quantified in five hemisegments per embryo in uninjected controls and netrin 1a morphants. (F) The fraction of intersomitic boundaries bearing secondary sprouts is not significantly changed in morphants. Uninjected controls, 75±5%; netrin 1a morphants, 61±8%. (G) Fewer secondary sprouts turn to form the PAC in morphants. Uninjected controls, 95±3%; netrin 1a morphants, 9±5%. All values are mean±s.e.m.; error bars show s.e.m.; P-value determined by Mann-Whitney U test; n.s., not significant; n, number of hemisegments (number of embryos). A, anterior; D, dorsal; L, lateral; DLAV, dorsal longitudinal anastomotic vessel; NT, neural tube; NC, notochord; 1°, primary sprouts; 2°, secondary sprouts. Scale bars: 50 μm. |
|
Muscle pioneers express netrin 1a along the HMS prior to and during PAC formation and are required for PAC formation. (A-B2) mRNA in situ hybridization (ISH) shows netrin 1a expression in MPs and neural tube (NT) at 22 and 32 hpf. (B2) Transverse section of zebrafish embryo in B at the level indicated. (C-H) The muscle pioneers (MPs) are the critical source of Netrin 1a for PAC formation. Treatment with 50 µM cyclopamine prevents nearly all MP formation and leads to failure of PAC formation. 48 hpf fli1a:egfpy1 embryos antibody stained for vessels (GFP, green) and MPs (4D9 anti-engrailed, magenta). Confocal projections. (C-E) In DMSO-treated control embryos, MPs are present at the HMS (D) and the PAC forms normally (C, arrowheads). (F-H) In cyclopamine-treated embryos, very few MPs form (G) and the PAC is missing (F, arrowheads). (I) PAC formation was assessed in hemisegments 7-11 in DMSO-treated (97±2% complete; 3±2% partial; 0±0% absent) and cyclopamine-treated (21±6% complete; 15±4% partial; 64±7% absent) embryos. All values are mean±s.e.m.; P-value determined by Mann-Whitney U test comparing absent PACs. n, number of hemisegments (number of embryos). A, anterior; D, dorsal; NT, neural tube. Scale bars: 200 μm in A-B2 50 μm in C-H. EXPRESSION / LABELING:
PHENOTYPE:
|
|
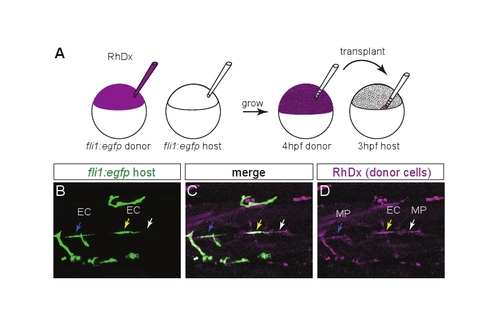
Transplanting wild-type MPs at the HMS rescues PAC formation in netrin 1a morphants. (A) Scheme for heterochronic transplants. Rhodamine Dextran (RhDx) is injected into one-cell stage fli1a:egfpy1 zebrafish embryos (donor). netrin1aSBMO is injected into one-cell stage fli1a:egfpy1 embryos (host). Cells are transferred from the RhDx embryo at 4 hpf to the netrin 1a morphant host at 3 hpf at a location fated to include MPs. (B-G) Lateral views showing fli1a:egfpy1 and the lineage marker (RhDx) in two transplanted embryos. The PAC (arrows, B,E) forms in the same hemisegments that receive transplanted wild-type MPs (arrowheads, C,F). Confocal projections. (H) Quantitation of rescue in netrin 1a morphants showing presence of the PAC (partial or complete) in hemisegments that either received wild-type transplanted MPs or were populated only by morphant host MPs. Host MPs only, 42±9%; transplanted donor MPs, 78±10%. All values are mean±s.e.m.; P-value determined by Wilcoxon signed rank test. n, number of hemisegments (number of embryos). A, anterior; D, dorsal; MP, muscle pioneer. Scale bar: 50 μm. |
|
dcc is required for turning of secondary sprouts at the HMS and injection of dcc mRNA rescues PAC formation in dcc morphants. (A-B2) 48 hpf fli1a:egfpy1 zebrafish embryos injected with (A,A2) plcg1 splice-blocking MO (plcg1SBMO) only (control) or (B,B2) plcg1SBMO plus dcc translation-blocking MO (dccTBMO). (A) Lateral view. In plcg1 morphants, secondary sprouts grow from the PCV and turn at the HMS to form the PAC (arrowhead). (A2) Transverse volume rendering of A showing mediolateral turn of secondary sprout. (star). (B) Lateral view. In embryos co-injected with plcg1SBMO and dccTBMO, secondary sprouts form but fail to turn and form the PAC (arrowheads). (B2) Transverse volume rendering of B showing failure of secondary sprout to turn laterally (star). (C) Secondary sprouts formed from the PCV were counted in 4-5 intersomitic boundaries per embryo between segments 7-11 in plcg1 single morphants (control, 97±3%) and in plcg1SBMO/dccTBMO double morphants (dccTBMO, 90±5%), and showed no significant difference (n.s.). (D) Secondary sprouts that turned to form the PAC were counted in plcg1 single morphants (87±5%) and plcg1/dcc double morphants (18±5%), which showed greatly reduced turning in the absence of Dcc. (E,F) Lateral views of 48 hpf fli1a:egfpy1 embryos injected with (E) dccTBMO or (F) dccTBMO and dcc mRNA. The PAC is absent in dcc morphants (E, arrowheads) and restored when dcc morphants are co-injected with dcc mRNA (F, arrow and arrowheads indicate complete and partial rescue, respectively). (G) Quantification of complete, partial or absent PAC. Uninjected: complete, 99±1%; partial, 1±1%; absent, 0±0%. dcc morphant: complete, 31±7%; partial, 25±6%; absent, 44±7%. dcc morphant + RNA: complete, 86±5%; partial, 9±4%; absent, 5±3%. All values are mean±s.e.m.; error bars show s.e.m.; P-value determined by Mann-Whitney U test (comparing absent PACs in G). n, number of hemisegments (number of embryos). A, anterior; D, dorsal; L, lateral; PAC, parachordal chain. Scale bars: 50 μm. |
|
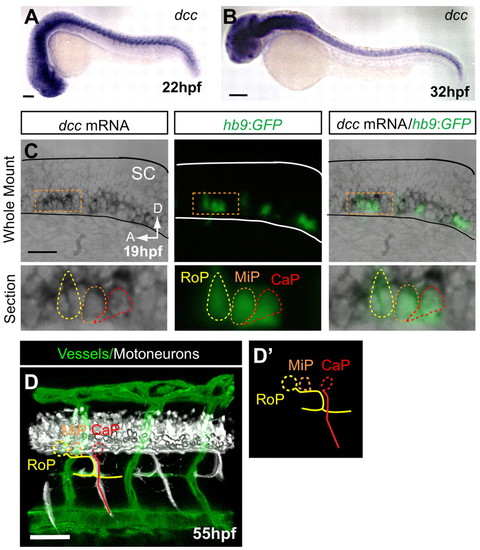
dcc is not detected in endothelial secondary sprouts but is expressed in motoneurons with axons present at the HMS prior to PAC formation. (A,B) dcc mRNA is expressed in the ventral spinal cord at 22 and 32 hpf and is not detectable in the secondary sprouts or other trunk vasculature. (C) Whole-mount and longitudinal section of hb9:GFP transgenic zebrafish embryo showing dcc mRNA ISH (purple), where hb9:GFP labels only motoneurons at early stages (green). (D) Lateral volume rendering of fli1a:dsRedEx; hb9:GFP embryo at 55 hpf. Double transgenic labels endothelial cells (green) and motoneuron axons (white) in the trunk. Rostral primary motoneuron (RoP, yellow) axons are present at the HMS adjacent to the PAC. (D2) Outline of motoneurons alone. CaP, caudal primary motoneuron (red); MiP, middle primary motoneuron (orange; axon omitted for clarity). SC, spinal cord; A, anterior; D, dorsal. Scale bars: 100 μm. EXPRESSION / LABELING:
|
|
RoP axons and associated secondary axons at the HMS do not form in netrin 1a and dcc morphants. (A) Diagram of RoP, middle primary (MiP) and caudal primary (CaP) axons at 36 hpf. (B-D) Lateral view of 55 hpf hb9:GFP zebrafish embryos injected with (B) no MO, (C) netrin 1a splice-blocking MO (netrin1aSBMO) or (D) dcc translation-blocking MO (dccTBMO). Confocal projections. (B) Uninjected controls show axons at the HMS in almost every somite (arrowheads). In embryos injected with (C) netrin1aSBMO or (D) dccTBMO, axons fail to form at the HMS (arrowheads). (E) Axons at the HMS were counted in 3-4 somites per embryo in segments 7-11 in control, netrin1aSBMO- and dccTBMO-injected embryos. Uninjected, 97±3%; netrin 1a morphant, 27±7%. Uninjected, 98±2%; dcc morphant, 49±7%. All values are mean±s.e.m.; P-value determined by Mann-Whitney U test. n, number of hemisegments (number of embryos). A, anterior; D, dorsal; L, lateral; RoP, rostral primary motoneuron; MiP, middle primary motoneuron; CaP, caudal primary motoneuron. Scale bars: 50 μm. |
|
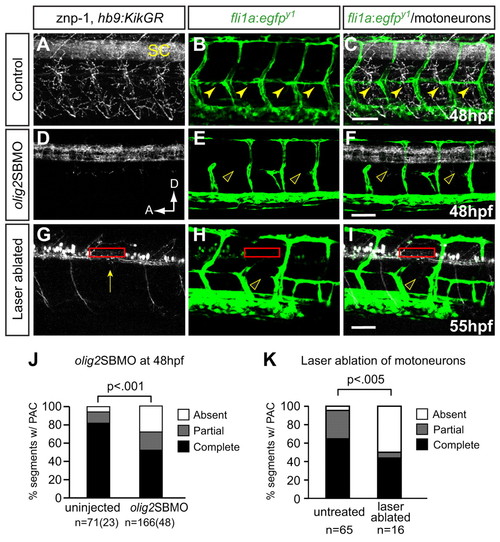
Preventing differentiation or laser ablation of motoneurons prevents PAC formation. (A-F) Lateral views of 48 hpf fli1a:egfpy1 zebrafish embryos either uninjected or injected with olig2 splice-blocking MO (olig2SBMO) showing labeled vessels (green) and neurons (znp-1 antibody, white). Confocal projection. (A) Motor axons grow ventrally from the spinal cord (SC) in controls. (B,C) The PAC forms normally in controls (arrowheads). (D) Motoneurons and their axons are absent in olig2 morphants. (E,F) PAC does not form in olig2 morphants (arrowheads). (G-I) Lateral view of 55 hpf fli1a:egfpy1; hb9:KikGR after photoconversion, with vessels in green and motoneurons in white. Confocal projections. Motoneuron cell bodies were targeted by laser in one somite per embryo (red rectangle), resulting in ablation of motoneurons, (G) missing caudal primary motoneuron (CaP) axon (arrow), and (H,I) missing PAC (arrowhead) in that somite. (J) The PAC was scored in 4-5 somites per embryo in uninjected control embryos (complete, 82±6%; partial, 12±6%; absent, 6±4%) and olig2 morphants (complete, 52±5%; partial, 20±3%; absent, 28±4%). All values are mean±s.e.m.; P-value determined by Mann-Whitney U test. n, number of hemisegments (number of embryos). (K) The number of somites with PACs was quantified in somites that were untreated or successfully laser ablated. Untreated: complete or partial, 95%; absent, 5%. Ablated: complete or partial, 50%; absent, 50%. n, number of hemisegments; P-value determined by Fischer′s exact test. A, anterior; D, dorsal. Scale bars: 50 μm. |
|
netrin 1a and dcc morphants do not form a complete thoracic duct. (A,B,D,E) 5 dpf fli1a:egfpy1 larvae; reversed contrast, confocal projections. (A2,B2,D2,E2) Enlargements of the boxed regions from A,B,D,E. (A-A2,D-D2) The thoracic duct (TD) forms normally (arrows) between the dorsal aorta (DA) and posterior cardinal vein (PCV) in uninjected control embryos. (B,B2,E,E2) The TD is absent in netrin1aSBMO or dccTBMO morphants (open arrowheads). (C,F) Quantitation of partial or complete TD formation in control embryos (77%), netrin1aSBMO morphants (25%) and dccTBMO morphants (45±19%; mean±s.e.m.); P-values, Mann-Whitney U test. n, number of hemisegments (number of embryos). PAC, parachordal chain; DA, dorsal aorta; PCV, posterior cardinal vein. Scale bars: 50 μm. |
|
netrin 1a is required for secondary sprout elongation along the horizontal myoseptum. Selected frames spanning 8 hours of live time-lapse imaging of (A-E) a plcg1y10; fli1a:egfpy1 embryo and (F-J) a plcg1y10; fli1a:egfpy1 embryo injected with netrin1aSBMO1. Lateral views, confocal z-projections spanning both left and right sides. (A-E) In uninjected embryos, secondary sprouts have reached the horizontal myoseptum (HMS) and soon thereafter start to elongate along the anterior-posterior axis (arrows). (F-J) In the netrin 1a morphant, secondary sprouts have reached the HMS but fail to elongate anteroposteriorly (arrows). A, anterior; D, dorsal. Scale bar: 50 μm. |
|
netrin 1a expression at the HMS is abolished in embryos treated with 50 µM cyclopamine. Lateral view of in situ hybridizations at 32 hpf. (A) In 1% DMSO-treated controls, netrin 1a is expressed in the neural tube (NT) and muscle pioneers (MP) at the HMS (arrows). (B) netrin 1a is expressed in the NT but lost at the HMS (red arrowhead) in embryos that lack MPs following cyclopamine treatment. NT, neural tube; MP, muscle pioneer. Scale bar: 100 μm. |
|
Distinguishing between transplanted muscle pioneers and transplanted endothelial cells. (A) Heterochronic transplant scheme used for this test: donor is labeled with Rhodamine Dextran (RhDx) and both donor and host are marked with fli1a:egfpy1. (B-D) Live lateral views of a 48 hpf transplanted embryo. There are three classes of RhDx-positive cells at the HMS. (1) A RhDx+ GFP- cell. This is a transplanted muscle pioneer (MP, white arrow). (2) A RhDx+ GFP+ cell in which the GFP and RhDx fluorescence coincide. This is a transplanted endothelial cell (EC, yellow arrow). (3) A RhDx+ cell adjacent to, but not coinciding with, a GFP+ cell. This is a transplanted MP adjacent to a host EC (blue arrow). For rescue experiments in which the host was not marked with fli1a:egfpy1, class 3 combines with class 1; however, it is still possible to distinguish between transplanted MPs and transplanted ECs by looking for coincident GFP expression from the donor fli1a:egfpy1 transgene. |
|
dccTBMO morphants and isl1 morphants form MPs and express netrin 1a at the HMS. (A,C,E) 32 hpf fli1a:egfpy1 embryos were antibody stained for MPs (4D9 anti-engrailed, red). (B,D,F) mRNA in situ hybridization for netrin 1a expression in 32 hpf embryos. (A,C,E) MPs are present at the HMS in (A) uninjected control embryos, (C) dcc morphants and (E) isl1 morphants. (B,D,F) netrin 1a mRNA expression is present at the HMS (arrows) in (B) control embryos, (D) dcc morphants and (F) isl1 morphants. MPs, muscle pioneers; A, anterior; D, dorsal. Scale bar: 100 μm. |
|
Isl1 mutants and morphants form secondary sprouts but they do not turn to form a parachordal chain. (A-H) Lateral views of 48 hpf isl1+/?; fli1a:egfpy1 or isl1-/-; fli1a:egfpy1 embryos injected with plcg1 morpholino and stained with znp-1 antibody. Confocal projections. (D,H) Volume renderings of a single hemisegment, transverse view. (A-D) In plcg1 morpholino-injected isl1+/? sibs, motoneuron axons form (A), secondary sprouts grow from the posterior cardinal vein (PCV) (B) and turn at the HMS (filled star, D) to form the PAC (parachordal chain, solid yellow arrowheads, B). (E-H) In plcg1 morpholino-injected isl1-/- mutants, secondary sprouts form and extend dorsally (F) but fail to turn (star, H) and form the PAC (arrowheads, F). (I) Quantitation of PAC formation in 4-5 somites per embryo of plcg1 morpholino-injected isl1 mutants and siblings. plcg1/isl1+/?: complete, 60±16%; partial, 15±8%; absent, 25±13%. plcg1/isl1-/-: complete, 25±15%; partial, 42±4%; absent, 71±14%. (J-L) Lateral views of 48 hpf fli1a:egfpy1 embryos injected with isl1 morpholino and stained with znp-1 antibody. Confocal projections. In isl1 morphants, motoneuron axons form (J) and the PAC does not form (arrowheads, K). (M) Quantitation of PAC formation in 4-5 somites per embryo. Uninjected control embryos: complete, 91±4%; partial, 9±4%; absent, 0±0%. isl1 morphants: complete, 26±6%; partial, 32±8%; absent, 42±10%. All values are mean±s.e.m.; P-values by Mann-Whitney U test. n, number of hemisegments (number of embryos). A, anterior; D, dorsal; L, lateral. Scale bars: 50 μm. |