- Title
-
Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin
- Authors
- Rieger, S., and Sagasti, A.
- Source
- Full text @ PLoS Biol.
|
(A) A transient transgenic larva expressing GFP in two RB neurons: one innervating the trunk (red arrowhead) and one innervating the tail fin (white arrowhead). Arrow indicates the RB peripheral arbor in the tail fin. (B) Fin amputation in a 78 hpf islet2b:GFP transgenic larva caused severed axon branches to degenerate at the wound margin (brackets, 1 h post-amputation [hpamp]). RB axon arbors completely reinnervated the regenerated fin by 120 hpamp. (C, D) Time-lapse sequences from 78–90 hpf. The rightmost panel shows axon tip trajectories (red) over the course of the time-lapse (see also Video S1 for a tracing example). (C) The branches of a single GFP-labeled peripheral axon in an uninjured fin underwent minimal growth and retraction. Some of this apparent activity was due to movement of the tissue during time-lapse (see Video S1). (D) Fin amputation (dotted line) increased the growth of the severed arbor (see also Figure 3D, E) and promoted reinnervation of denervated territory (shaded area) (Video S3). (E) Quantification of axon activity in uninjured fins (n = 13) and after fin amputation (n = 26) (two-tailed, unpaired Student′s t-test, *** p<0.001). Error bars represent the standard error of the mean. hpamp, hours post amputation. |
|
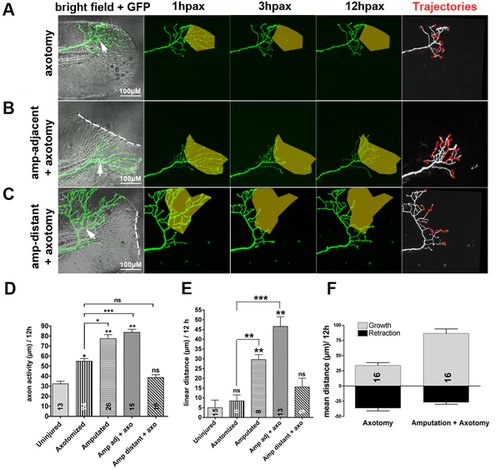
(A–C) Time-lapse sequences from 78–90 hpf. The rightmost panel shows axon tip trajectories (red) over the course of the time-lapse. (A) Axotomy (arrow) without amputation (see also Video S2). Axotomy increased axon activity, but axons were unable to reinnervate denervated territory (shaded area) (Figure S2). (B) The ability of an axotomized arbor (arrow) to reinnervate denervated territory (shaded area) was improved by fin amputation (dotted line) (see also Figure S2 and Video S4). (C) Axotomized arbors distant from the amputation plane did not regenerate (see also Video S5). (D, E) Comparison of axon activity (growth and retraction) (D) and linear growth distance (E) in uninjured arbors, after axotomy alone, after amputation alone, or after axotomy and amputation, both in close proximity (adj) or at a distance of greater than 50 µm. (F) Comparison of growth and retraction between axotomized axons, and axotomized axons in larvae whose fins were also amputated. Amputation in addition to axotomy promoted a shift from balanced growth and retraction toward growth. Sample size for each group is indicated by the number in the bar. Error bars represent the standard error of the mean. For statistical analyses, we performed one-way ANOVA and either Dunnett′s post-test to compare individual groups to uninjured controls (asterisks above bars indicate significance compared to control) or Bonferroni′s post-test to compare individual groups with each other (as indicated by brackets, p = ns>0.05, * p<0.05, ** p<0.01, *** p<0.001). hpax, hours post axotomy; amp, amputation. |
|
Time-lapse sequences from 78–90 hpf. The rightmost panel shows axon tip trajectories (red) over the course of the time-lapse. (A) Ablating muscle cells (circles) in the fin of a transgenic reporter larva was accompanied by only limited regeneration of an axotomized arbor (arrow). (B) Ablating e3 keratinocytes (red) (circles) in the fin promoted axon regeneration after axotomy (arrow) and improved reinnervation of denervated territory (shaded area). (C) Axotomy of a trigeminal axon branch in the head (arrow) induced limited growth of the severed axon, but the denervated territory was avoided (shaded area). (D) Ablation of e3 keratinocytes and axotomy of a trigeminal axon (arrow) promoted robust growth of the severed axon and reinnervation of the denervated territory (shaded area). (E) Quantification of axon activity after keratinocyte and muscle cell ablations. Sample size for each group is indicated by the number in the bar. Error bars represent the standard error of the mean. For statistical analyses, we performed one-way ANOVA and Dunnett′s post-test to compare individual groups to control groups (ablation of 1 keratinocyte in the fin or the head) (asterisks above bars indicate significance compared to control) (p = ns>0.05, ** p<0.01, *** p<0.001). krtc, keratinocyte. |
|
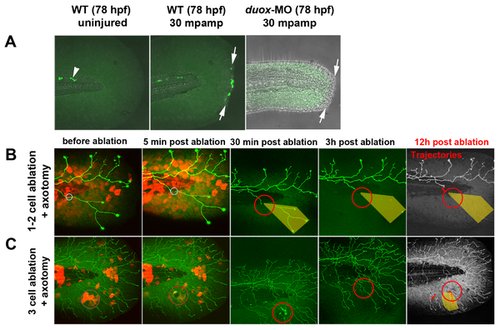
(A) In uninjured fins, H2O2 was always detectable with pentafluorobenzenesulfonyl fluorescein (see Methods) in a few cells near the notochord (arrowhead) but was not detectable in fin tissue. Fin amputation (arrows) produced high levels of H2O2 in wound marginal cells at 30 min post-amputation. In contrast, H2O2 levels were either undetectable or very low in duox1 morphants at 30 mpamp. (B, C) Ablating multiple keratinocytes produced hydrogen peroxide (H2O2). Time-lapse sequences from 78–90 hpf. The rightmost panel shows axon tip trajectories (red) over the course of the time-lapse and denervated territory (shaded areas). (B) Ablation of one or two keratinocytes (circles) did not produce sufficient H2O2 to be detected by the H2O2 sensor pentafluorobenzenesulfonyl fluorescein and did not promote axon regeneration of a nearby severed axon. (C) Ablating more (e3) keratinocytes (circles) induced H2O2 production around the wound margin, as detected with the H2O2 sensor, and improved regeneration of a nearby axotomized arbor, which grew into denervated territory (shaded area, trajectories). mpamp, minutes post amputation. |
|
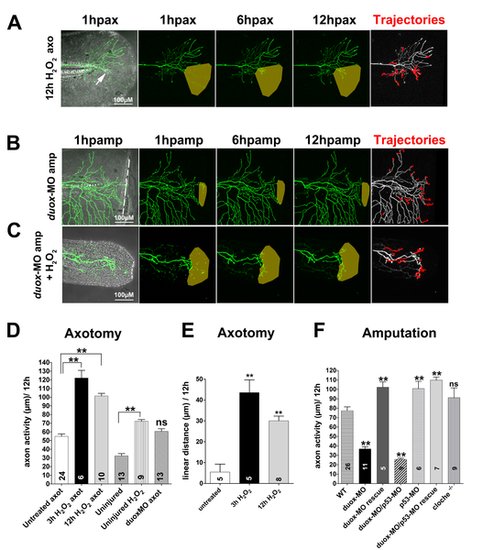
Time-lapse sequences from 78–90 hpf. The rightmost panel shows axon tip trajectories (red) over the course of the time-lapse; denervated territories are indicated by shaded areas. (A) Adding 3 mM H2O2 to the larval media for 12 h enhanced axon growth and promoted reinnervation of denervated territory after axotomy (arrow) in a non-amputated fin (see also Video S6). (B) Fin amputation did not promote axon growth in a duox1-morphant larva. The axon trajectories reflect mostly tissue movement during the time-lapse (see Video S7). (C) Adding 1.5 mM H2O2 rescued axon growth and improved reinnervation of denervated territory in amputated duox1-morphants (see also Video S8). (D, E) Quantification of axon activity (D) and linear axon growth (E) after axotomy in larvae treated with H2O2. (F) Quantification of axon activity after fin amputation. Sample size for each group is indicated by the number in the bar. Error bars represent the standard error of the mean. For statistical analyses, we performed one-way ANOVA and either Dunnett′s post-test to compare individual groups to controls (asterisks above bar indicate significance compared to control, the first column in each graph) or Bonferroni′s post-test to compare individual groups with each other (as indicated by brackets, p = ns>0.05, ** p<0.01). axo, axotomy; amp, amputation. |
|
(A) Diagram of the procedure for creating chimeric larvae to test where Duox1 functions to promote axon regeneration: cells from a duox1-MO injected sensory:GFP transgenic donor were transplanted into a wildtype Krt4:RFP transgenic host. GFP-labeled Rohon-Beard sensory neurons were thus deficient in Duox1 function, while RFP-labeled keratinocytes were wildtype. (B) Ablation of e3 wildtype keratinocytes (circle) and axotomy of a nearby duox1-morphant RB axon in the upper trunk region (arrow) promoted regeneration of severed axon branches (arrowheads) and reinnervation of denervated territory (shaded area) (see also Video S9). (C) Quantification of axon activity in chimeric embryos after keratinocyte ablation and axotomy, compared to basal growth (same as in Figure 1E), axotomy alone (same as in Figure 3D), and keratinocyte ablation (same as in Figure 4E). Sample size for each group is indicated by the numbers in the bars. Error bars represent the standard error of the mean. For statistical analyses, we performed one-way ANOVA and either Dunnett′s post-test to compare individual groups to controls (asterisks above bars indicate significance compared to control, the first column in graph) or Bonferroni′s post-test to compare individual groups with each other (as indicated by brackets, p = ns>0.05, * p<0.05, *** p<0.001). |
|
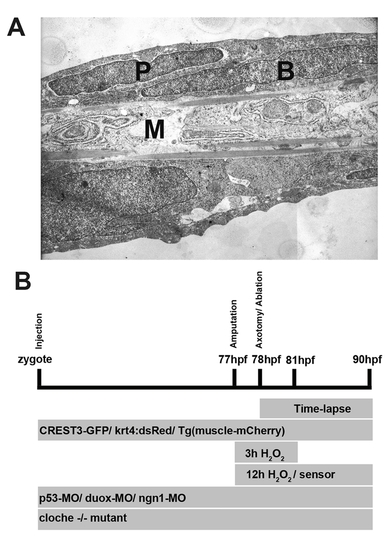
Ultrastructure of a larval fin and experimental design. (A) Transmission electron micrograph of a sagittal section through the caudal fin at 48 hpf. The skin consists of two cell layers, the outer periderm (P) and inner epidermal basal cells (B), which are separated by a basement membrane from medially located muscle (M). Magnification is 4,800×. (B) Timeline of experimental procedures. hpf, hours post fertilization. |
|
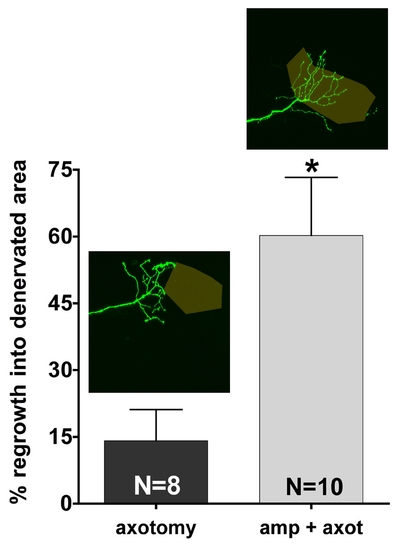
Quantification of peripheral RB sensory axon reinnervation of denervated territories in the caudal fin. Example tracings are indicated above the bars (see Figure 3 and methods for details). Reinnervation was significantly increased when an axon branch was axotomized after fin amputation as compared to axotomy in non-amputated fins (60.24±13.06 μm versus 14.11±7.02 μm, * p<0.05; unpaired, two-tailed Student′s t-test). |
|
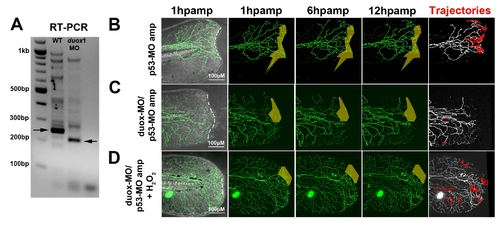
Knockdown of duox1 blocks the growth-promoting effects of amputation in p53 morphant larvae. (A) RT-PCR showing knockdown of duox1 wildtype transcript after morpholino injection as in [13]. Arrows point to the relevant bands. (B–D) Time-lapse sequences from 78–90 hpf. The rightmost panel shows axon tip trajectories (red) over the course of the time-lapse; denervated territories are indicated by shaded areas. (B) Enhanced axon growth in a p53 control-MO-injected larval fin after amputation (dotted line) and reinnervation of denervated territory (shaded area). (C) Co-injection of p53-MO and duox1-MO prevented axon growth and reinnervation after amputation. (D) Rescue of axon growth inhibition and reinnervation in p53-MO/duox1-MO double morphants in the presence of 1.5 mM H2O2. See quantification in Figure 6F. |