- Title
-
Topoisomerase II{beta} is required for lamina-specific targeting of retinal ganglion cell axons and dendrites
- Authors
- Nevin, L.M., Xiao, T., Staub, W., and Baier, H.
- Source
- Full text @ Development
|
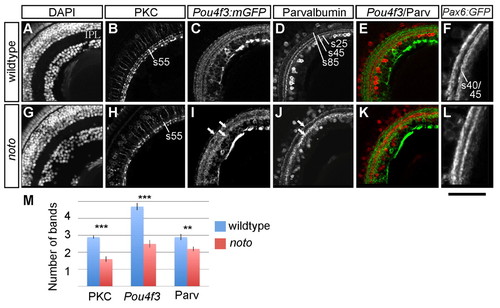
Fine neurite targeting is disrupted in the 5 dpf noto mutant inner plexiform layer (IPL). (A-L) Representative sections of wild-type (A-F) and mutant (G-L) zebrafish retina. DAPI nuclear stain shows the cellular organization of the retina, including location and width of the IPL (A,G). Protein kinase C (PKC)+ bipolar cells (BCs) project terminals to three IPL sublaminae in wild type (B); in the mutant, outermost sublamina s55 is sparsely innervated (H). Pou4f3:mGFP+ ganglion cell (GC) dendrites innervate five sublaminae in wild type (C); arrows in I show three coarse sublaminae in the mutant IPL (I). Parvalbumin (Parv)+ amacrine cells of the inner nuclear layer (INL) and ganglion cell layer (GCL) project neurites to three sublaminae (s25, s45 and s85) in wild type (D); arrows in J show two broad sublaminae in the mutant (J). Merged images show the relative positions of Pou4f3:mGFP+ and Parv+ IPL sublaminae (E,K). Pax6:mGFP+ amacrine cells project neurites to two IPL bands; the inner band is actually the two fine sublaminae s40 and s45 (F,L). The s40/s45 division is lost in the mutant. Scale bar: 50 µm for A-E,G-K; 30 μm for F,L. (M) Mean number of distinct bands of PKC, Pou4f3:mGFP and Parv immunoreactivity from ten wild-type (blue) and ten mutant (red) retinas. Error bars represent s.e.m. **P<0.01; ***P<0.00001. |
|
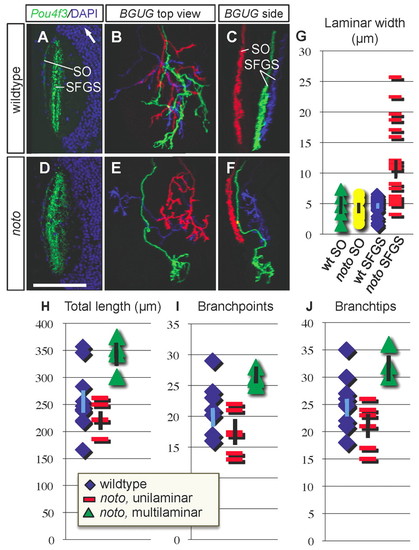
noto mutant ganglion cell (GC) axons project aberrantly between SFGS tectal sublaminae. (A,D) Representative horizontal sections of wild-type (A) and mutant (D) tectum in Pou4f3:mGFP+ 5 dpf zebrafish larvae. GFP is in green, DAPI nuclear stain is in blue. White arrow in A points rostrally. (B,C,E,F) Small numbers of GC axons labeled in BGUG+ transgenic wild-type (B,C) and mutant (E,F) larvae. Images are projections of confocal stacks in top (B,E) and side (C,F) views. Each red axon lies in SO; blue and green axons innervate SFGS. Both green and blue axons in panel F are wider than 10 µm in side view and scored as multilaminar. Scale bar: 70 μm for A,D; 50 μm for B,C,E,F. (G) Scatter graph of side-view widths of wild-type SO (n=4), mutant SO (n=6), wild-type SFGS (n=24) and mutant SFGS (n=24) axons in wild type and mutant. (H-J) Morphometric analysis of wild-type and mutant BGUG+ GC axons. Scatter plots showing total arbor length (H), number of branchpoints (I) and number of branchtips (J) in wild-type (n=10), mutant unilaminar (n=5) and mutant multilaminar (n=3) SFGS axons. Population means are plotted as points, error bars represent s.e.m. |
|
noto mutant tectal neurons have unrefined neuropil projections at 5 dpf. Sections of wild-type (A,C,E) and mutant (B,D,F) zebrafish tectum showing tectal cell populations and their neuropil neurites. Disorganized neuropil in the mutant is marked with asterisks. (A,B) Wild-type Parvalbumin (Parv)+ tectal neurons project neurites to four laminae in the neuropil; this laminar organization is incomplete in the mutant. (C,D) Wild-type Protein kinase C (PKC)+ tectal neurons project neurites to three laminae in the neuropil; this laminar organization is lost in the mutant. (E,F) Merged images showing relative location of laminae. Scale bar: 50 μm. |
|
The noto genetic lesion is a nonsense splice site mutation in top2b. (A) Sequence chromatograms showing the top2b exon 11 splice acceptor site in wild-type and mutant zebrafish. The sequence shown is at the intron-exon border shown in yellow and blue, respectively. (B) Schematic of the functional domains of Top2b protein. Solid colored regions are preserved in the predicted mutant protein; diagonal lines indicate the truncated portion. (C) RT-PCR-amplified top2b fragments from uninjected, control morphant and top2b morphant fish at 2 and 5 dpf. Morpholino oligonucleotide (MO) knockdown results in a truncated cDNA; both the full-length and truncated products can be detected by 5 dpf. (D-G) Ganglion cell (GC) axons from BGUG+ larvae in morpholino phenocopy experiment. (D,E) Top (D) and side (E) views of two SFGS axons from a control morphant. (F,G) Top (F) and side (G) views of three SFGS axons from an antisense top2b morphant; red axon is multilaminar. Scale bar: 50 μm. (H-K) In situ hybridization showing expression of top2b in 4 dpf larvae. (H) Antisense probe labels brain and eye. Unlabeled larvae are homozygous wild type (+/+), single asterisk indicates noto+/-, double asterisk indicates noto-/-.(I) Sense probe labeling is minimal. (J) Magnified dorsal view of antisense labeling. (K) Higher magnification view of sectioned retina, showing strong expression in amacrine cells (ACs) and GCs. Scale bar: 250 μm for H,I; 150 μm for J; 50 μm for K. |
|
Expression patterns of early brain and eye development are preserved in noto mutants. (A,B) In situ hybridization with ath5 antisense probe at 28 hpf shows normal progression of neurogenesis in the wild-type and mutant zebrafish retina. Border of eye, lens and position of the choroid fissure (CF) are indicated by dashed lines. (C,D) pax2a expression at 28 hpf shows grossly normal development of the optic stalk (OS), midbrain-hindbrain boundary (MHB) and neurons of the hindbrain (HB) in both wild type and mutant. (E,F) cntn2 expression at 36 hpf shows grossly normal development of the forebrain (FB), several cranial ganglia (CG), the cerebellum (CB) and hindbrain (HB) in both wild type and mutant. (G,H) Confocal images of whole-mount HuC/D antibody-stained 48 hpf embryos. A similar population of HuC/D labeled ganglion cells (GCs) has developed in wild type (G) and mutant (H). Scale bar: 40 μm for A,B; 100 μm for C-F; 30 μm for G,H. |
|
noto mutant ganglion cell (GC) sublamination defects are retina-autonomous. (A-D) GC axons derived from wild-type zebrafish donors, innervating mutant tecta. (A,B) Top and side views of a large cluster of axons, including one SO and many SFGS axons, showing orderly sublamination. (C,D) Top and side views of individual GC axons, showing fine sublamination within SFGS. (E-H) GC axons derived from mutant donors, innervating wild-type tecta. (E,F) Top and side views of a large cluster of SFGS axons, showing disordered sublamination; arrows show laminar crossovers. (G,H) Top and side views of three SFGS axons. Red axon is multilaminar; arrow shows crossover. Scale bar: 50 μm. |
|
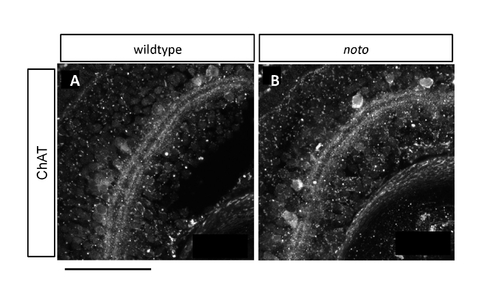
Choline acetyltransferase (ChAT)+ amacrine cells have incomplete fine targeting in noto mutants. (A,B) Sections of 5 dpf wild-type (A) and mutant (B) zebrafish retina, immunostained for ChAT. In wild type, the two inner plexiform layer (IPL) bands are each doublets; in the mutant these are single bands. Scale bar: 50 μm. |
|
Inner plexiform layer (IPL) sublamination defects in noto mutants become more severe by 7 dpf. (A,B) Protein kinase C (PKC)+ bipolar cells project terminals to three IPL sublaminae in wild type (A), whereas in the mutant (B) this targeting is coarse and the outermost sublamina remains sparse. (C,D) Pax6:mGFP+ neurites from the same sections shown in A and B, showing severely coarse targeting in the mutant. (E,F) Merged images showing the relative location of sublaminae. (G,H) Parvalbumin (Parv)+ amacrine cells project neurites to three IPL sublaminae in wild type (G), whereas in the mutant (H) this targeting has become very coarse. (I,J) Pax6:mGFP+ neurites from the same sections shown in G and H, showing severely coarse targeting in the mutant. (K,L) Merged images showing the relative location of sublaminae. Scale bar: 50 μm. |
|
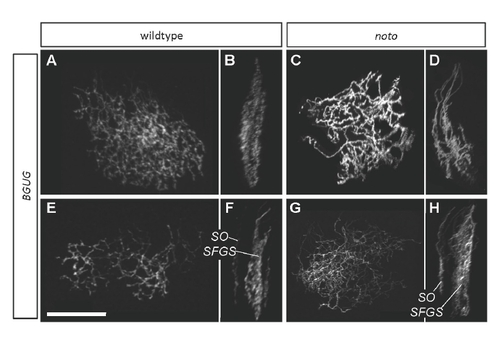
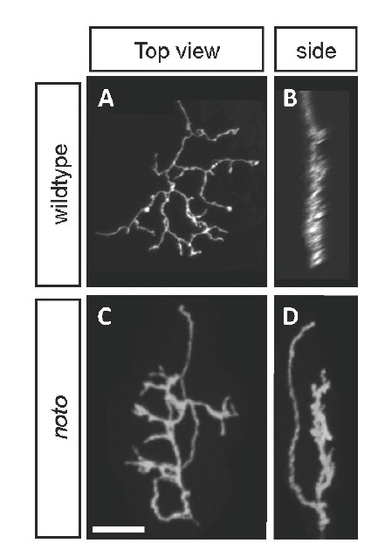
BGUG+ ganflion cell (GC) axons are disorganized in 5 dpf noto mutant tecta. Images are projections of confocal stacks collected from live transgenic larvae. (A,B) Several wild-type SFGS GC axons in top (A) and side (B) views. (C,D) A similar number of mutant SFGS axons. Crossovers between sublaminae are evident. (E,F) Top and side views of a large group of wild-type GC axons, including one innervating SO. SO and SFGS axons are clearly separated. (G,H) A similar number of mutant axons, including one innervating SO. SFGS disorganization is apparent, and a dimly labeled axon appears to cross between SO and SFGS. This was the only observed instance of an SO-SFGS crossover. Scale bar: 50 μm. |
|
Representative entry-defective ganglion cell (GC) axon in noto mutants at 5 dpf. (A,B) Typical wild-type axon imaged live from a BGUG+ larva, in top (A) and side (B) views. (C,D) Entry-defective GC axon in a BGUG+ mutant larva. Initial axon segment lies in distinct sublamina from branched arbor; axon makes a 180° turn and enters a new sublamina before branching. Scale bar: 15 μm. |
|
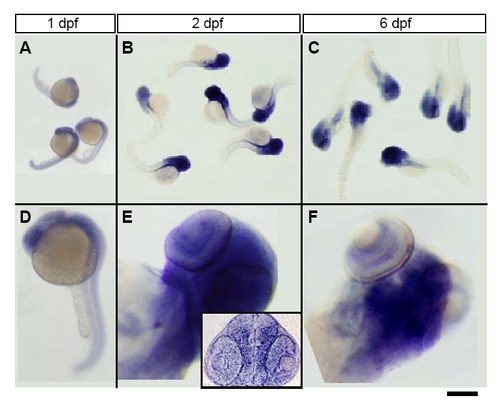
Time course of top2b expression. Top row shows noto clutch embryos and larvae stained with top2b antisense probe. Bottom rows shows higher power images of single fish. (A,D) top2b is expressed ubiquitously at 1 dpf. (B,E) By 2 dpf, expression is restricted to the eyes, head and trunk. Inset in E shows a coronal section showing broad expression in eye and brain. (C,F) By 6 dpf, expression appears to be restricted to the eye and brain. Scale bar: 1 mm in A; 1.2 mm in B,C; 400 μm in D; 100 μm in E,F; 120 μm in E (inset). |