- Title
-
Roles of brca2 (fancd1) in Oocyte Nuclear Architecture, Gametogenesis, Gonad Tumors, and Genome Stability in Zebrafish
- Authors
- Rodriguez-Mari, A., Wilson, C., Titus, T.A., Cañestro, C., Bremiller, R.A., Yan, Y.L., Nanda, I., Johnston, A., Kanki, J.P., Gray, E.M., He, X., Spitsbergen, J., Schindler, D., and Postlethwait, J.H.
- Source
- Full text @ PLoS Genet.
|
Expression of brca2 in wild-type gonads. (A,B) 26dpf transitioning stage ovaries and testes, respectively, express brca2 in the ooplasm of oocytes (arrow) and in male germ cells. (C,D) Expression of brca2 increases in 33dpf immature ovaries and testis, respectively. Arrows indicate examples of brca2-expressing cells. (E) In wild-type ovaries, brca2 transcript is highly expressed in the ooplasm of late stage IB oocytes (L-IB, arrow) and gradually becomes localized to a small portion of the ooplasm in stage III and IV oocytes (arrowheads). (F) Spermatocytes contain brca2 transcript but spermatids and sperm do not. (G) Stage III-IV oocytes accumulate brca2 transcript in a pole of the oocyte. (H) Comparison to the adjacent section probed for the animal pole marker pou5f1(oct4) reveals that brca2 transcript localizes to the animal pole in the same location as pou5f1(oct4) message (arrows in G and H). Abbreviations: II, III, IV, stages of oocyte development; L-IB, late stage IB oocyte (stages according to [49]); do, degenerating oocyte; GV, germinal vesicle; sc, spermatocyte; sp, sperm. EXPRESSION / LABELING:
|
|
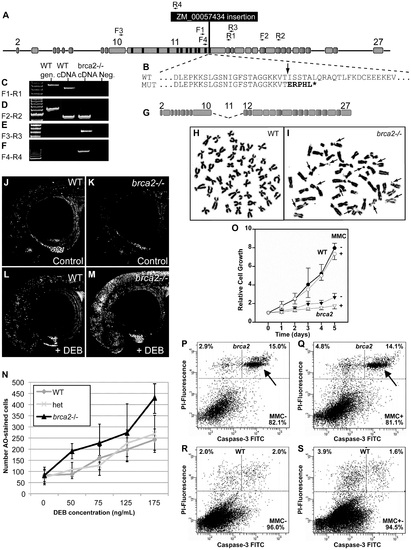
A zebrafish brca2 insertional mutant reveals a role for brca2 in zebrafish genome stability and cell growth. (A) The ZM_00057434 insertion disrupts exon-11 of zebrafish brca2 in the last BRC repeat. (B) The insertion (arrow) disrupts the wild-type (WT) sequence, leading to a mutant (MUT) sequence that contains five substituted amino acid residues encoded by the insert followed by a stop codon (*) that truncates the protein before the DNA binding domain, which is essential for Brca2 activity. (C) Amplification using primers F1 and R1 (see panel A and Table S1) produced a transcript from wild-type cDNA but none from homozygous mutant cDNA. (D) Amplification using primers F2 and R2 showed that transcripts including exon-18 occur in both wild types and mutants. (E) Amplification using primers F3 and R3 revealed that mutants, but not wild types, made transcripts that lack exon-11. (F) Amplification using primers F4 and R4 showed that mutants, but not wild types, made transcripts that include part of exon-11 and the insert. (G) The transcript amplified in panel F is predicted to make a transcript that lacks exon-11 but continues in-frame and makes a non-functional protein lacking all BRC repeats. (H) Most tissue culture cells established from the caudal fin of wild-type adults had a normal karyotype even after MMC treatment. (I) Most tissue culture cells established from the caudal fin of homozygous brca2 mutant adults showed chromatid breaks after MMC treatments, including acentric fragments, and radial chromosomes (arrows). (J,K) Acridine orange (AO) treatment of wild types and brca2 mutants not treated with DEB revealed about equal levels of cells staining with AO. (L,M) After DEB treatment, the number of AO-positive cells increased a small amount in 28hpf wild-type embryos but increased substantially in 28hpf brca2 mutant embryos. (N) The number of AO-positive cells increased with DEB concentration in all three genotypes tested, but nearly twice as much in brca2 mutant cells. (O-S) Growth, apoptosis and non-apoptotic cell death in cultured zebrafish fibroblasts. (O) Cell cultures established from brca2 mutant caudal fins (brca2, triangles) grew slower than cultures established from wild types (circles), reaching only 33.5% of the control count after 5 days. The additional effect of 15 nM mitomycin C (MMC) on growth retardation was relatively low but more distinct in brca2 mutant cultures than wild-type cultures (open triangles and open circles, respectively). The graph displays the mean and standard deviation of multiples of the seeded cell number from three independent experiments. (P-S) Apoptosis is mainly responsible for the growth deficit of brca2 mutant cells. (P) Untreated brca2 cultures showed spontaneous apoptosis of 15% of cells (upper right quadrant) and 2.9% of cells experienced non-apoptotic death (upper left quadrant). (Q) Exposure to 50 nM mitomycin C for 24 h increased the non-apoptotic cell death rate in brca2 mutant cultures only to 4.8%, whereas the rate of apoptotic cells remained about the same (14.1%). (R) Wild-type cultures revealed just 2% spontaneous apoptosis (upper right quadrant) and 2% non-apoptotic cell death (upper left quadrant). (S) Exposure to 50 nM mitomycin C for 24 h increased the non-apoptotic cell death rate to 3.9% but left the rate of apoptotic cells unchanged (1.6%). |
|
Developing gonads of brca2 mutants lack perinucleolar oocytes and develop testes that contain pyknotic and apoptotic cells. In transitioning gonads of wild types at 21dpf, ovary-like gonads (A) contained perinucleolar oocytes (early stage IB), while testis-like gonads (B) contained gonia, some early oocytes, and a few pyknotic cells. (C) Gonads of 21dpf brca2 mutants contained a few early oocytes and many pyknotic cells. (D) By 27dpf, perinucleolar oocytes had enlarged in wild-type ovaries. (E) Wild-type 27dpf testes possessed gonia. (F) 27dpf brca2 mutant gonads contained gonia, some early oocytes (judging from cell size and the location and number of nucleoli), showed testis-like morphology, and had many pyknotic cells. (G) At 32dpf, wild-type immature ovaries contained growing perinucleolar oocytes that had reached diplotene stage (late stage IB). (H) Wild-type 32dpf testes showed germ cells at all stages of spermatogenesis: spermatogonia, spermatocytes, spermatids, and sperm. (I) Homozygous brca2 mutant gonads at 32dpf possessed spermatogonia and spermatocytes, and tubules filled with pyknotic cells (dashed area) but lacked spermatids and sperm and had instead empty tubules (*). In transitioning gonads at 21–27dpf, we use the term ‘gonia’ (g) to represent cells that show the histological characteristics of spermatogonia (central nucleolus) and in differentiating testes at 32 dpf, we used the term ‘spermatogonia’ (sg) to represent such cells (Figure 4H′, 4I′). (J) Anti-active-Caspase-3 staining did not label cells in immature 60dpf wild-type testes. (K) In contrast, anti-active-Caspase-3 stained cohorts of cells in immature 60dpf mutant testes. (L,M) Staining the same sections with hematoxylin and eosin showed that cells staining for anti-active-Caspase-3 were pyknotic (arrows). Oocyte staging according to [49]. Magnification bar for A-I shown in A and for J-M shown in J. Abbreviations: asterisks (*), empty testis tubules; eo, early oocyte; pc, pyknotic cells; po, perinucleolar oocyte; sc, spermatocytes; sd, spermatids; sg, spermatogonia; sp, sperm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Adult brca2 mutant testes showed meiotic arrest and lack of spermatids and sperm and developed neoplasias. (A) Adult testes of wild types were organized into tubules that contained sperm (sp). (B) Adult testes of brca2 mutants were smaller in diameter and lacked sperm, but instead contained clusters of pyknotic cells and empty tubules (asterisks). (C) The anterior part of the testes (anterior testes) in wild-type adults possessed all stages of spermatogenesis: spermatogonia, spermatocytes at the bouquet stage (late zygotene-early pachytene) of meiosis, as well as later stages -- spermatids and sperm. (D,E,F) brca2 mutant anterior testes also had spermatogonia and bouquet stage spermatocytes, but strikingly, contained tubules of pyknotic cells, lacked spermatids and sperm, and had eosinophils that were not observed in wild-type testes. (G) The posterior part of the testes (posterior testes) of wild types contained tubules filled with germ cells at only the latest stage of spermatogenesis: sperm. (H) The posterior testis of brca2 mutants lacked sperm and had empty tubules (*) formed by interstitial cells. (I,J) The posterior testes of brca2 mutants developed neoplasias formed by spermatogonia and interstitial cells, demarcated by dotted lines and shown in enlargements (K,L). Abbreviations: asterisks (*), empty testis tubules; eos: eosinophils; ic: interstitial cells; pc: pyknotic cells; sc-b: spermatocytes at bouquet stage; sd: spermatids; sg: spermatogonia; sp: sperm. PHENOTYPE:
|
|
Altered Sertoli cell distribution, proliferation of spermatogonia, and accumulation of recombination-phase spermatocytes in brca2 mutant testes. In situ hybridization on adjacent sections of testes with markers for early germ cells (vasa), Sertoli cells (amh) and germ cells during recombination stages (sycp3) in wild types and brca2 mutants. (A-C) The anterior testis of wild types expressed vasa in early germ cells, amh in Sertoli cells surrounding tubules, and sycp3 in meiotic spermatocytes. (A′) Enlargement illustrates a gradient of vasa expression in spermatocytes: from moderate vasa expression (red circle) to low vasa expression (green circle), and finally spermatids and sperm (purple circle) that did not express vasa. (D) The anterior testis of brca2 mutants showed more tubules with vasa-expressing spermatogonia than wild types (A). (D′) Enlargement shows cells expressing low levels of vasa (red circle) and spermatocytes not expressing vasa (green circle) but no small spermatids or sperm. (E) brca2 mutants showed that amh-expressing cells were poorly organized and did not surround tubules neatly as in wild types, revealing an altered Sertoli cell distribution. (F) brca2 mutants expressed the recombination marker sycp3 in locally larger cell clusters than wild types, revealing an increased local concentration of cells at or entering pachytene stage. (G, H) In the posterior part of the testis, wild types did not express vasa or amh. (I) Hematoxylin and eosin (H&E) staining clearly showed sperm in many tubules in the posterior testes of wild types. (J-O) The posterior testes of brca2 mutants contained many tubules that were devoid of sperm (*) and contained vasa-expressing germ cells and Sertoli cells, which are not normally found in the wild-type posterior testes. (M-O) In one of two mutants analyzed, the posterior testes contained a larger proliferation of vasa-positive spermatogonia and also showed disorganized amh-expressing Sertoli cells, lacked sperm, and had empty tubules. Abbreviations: asterisks (*), empty testis tubules; gc, germ cells; i, intestine; ic, interstitial cells; sg, spermatogonia; sc, spermatocytes; Se, Sertoli cells; sp, sperm. |
|
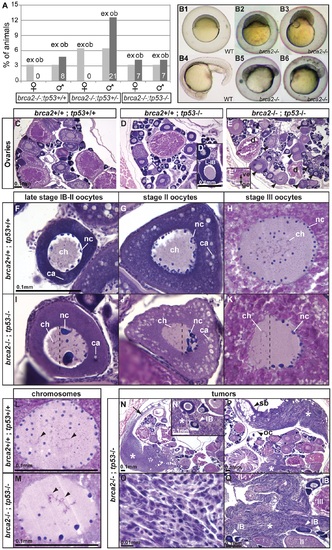
Mutation of tp53 rescued the brca2 sex reversal phenotype, yielding infertile double-mutant females that produced ovaries containing oocytes with altered nuclear architecture that developed into defective embryos and that produced invasive ovarian tumors. (A) Expected (ex) and observed (ob) frequencies of various tp53 genotypes among the homozygous brca2 mutant offspring of parents doubly heterozygous for tp53M214K and brca2 ZM_00075660 alleles. Females developed only in the absence of a wild-type tp53 allele, showing that the tp53 mutation rescued the sex reversal phenotype. (B) Doubly heterozygous embryos from the mating of homozygous tp53 females to males heterozygous for the brca2 mutation develop like normal embryos (B1 and B4 at 6 and 19 hpf, respectively) but the same genotype developing from the mating of rescued brca2;tp53 double mutant females and homozygous wild-type males stopped developing shortly after gastrulation (B2, B3, B5, B6). (C-E) Sections of ovaries from 6mpf wild-type, single mutant tp53, and double mutant brca2;tp53 adults, respectively, showed that oocytes in tp53 mutants developed normally (D′) but that oocytes degenerated and the vitelline envelope was abnormally separated from the granulosa cells in double mutants (E′). Strikingly, oocytes in double mutants showed nuclear anomalies (arrowheads in E). (F-H) Sections of wild-type late stage IB (transitioning to stage II), stage II, and stage III oocytes, respectively, contained radially symmetrical nuclei with small round nucleoli located mostly near the nuclear envelope and thin chromosomes separated from each other in the middle of the nucleus. (I-K) Sections of late stage IB, stage II, and stage III oocytes of brca2;tp53 double mutants showed abnormal nuclear asymmetry. Oocytes contained nuclei with large, variably-shaped nucleoli clustered at one side of the periphery of the nucleus instead of the normal uniform distribution around the periphery of the nucleus and chromosomes clustered at the other side of the nucleus opposite to the nucleoli. (L) Chromosomes in wild types were thin and separated (arrowheads). (M) Chromosomes in oocytes of double mutants were thicker and clumped and formed abnormal crosses and loops (arrowheads). (N,P) Invasive ovarian tumors (asterisks) were detected in sections of two of four brca2;tp53 double mutants by 6mpf but were not present in other genotypes (wild-types and single tp53 mutants). (N) One tumor appeared to originate at the ovarian cavity membrane (arrow) and the other tumor (P) was metastatic and contacted the ovarian cavity membrane and the swim bladder. (N′,Q) Both tumors were formed by spindle-shaped cells (O) that invaded the ovary and surrounded the oocytes. Abbreviations: IB,II,III: oocyte stages; ca, cortical alveoli; ch, chromosomes; d, degenerating oocyte; gc, granulosa cell; nc, nucleoli; oc, ovarian cavity; sb, swim bladder; ve, vitelline envelope. |
|
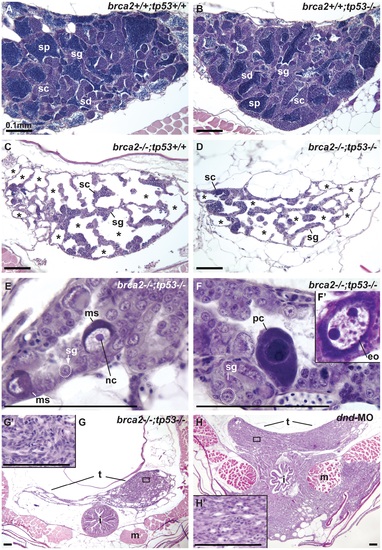
Mutation of tp53 failed to rescue infertility in brca2 mutants. (A,B) Wild-type testis and tp53 single mutant testis showed germ cells at all stages of spermatogenesis: spermatogonia, spermatocytes, spermatids and sperm. (C) brca2 single mutant testis contained spermatogonia and spermatocytes, lacked spermatids and sperm and had large regions devoid of germ cells (asterisks). (D) Testes in brca2;tp53 double mutants were similar to the brca2 single mutant, but had additional developmental problems. (E) Double mutant testis formed large cells with a single central nucleolus like spermatogonia (called here megalospermatogonia). (F) Some of these abnormally enlarged cells were pyknotic and some showed an oocyte-like morphology similar to early oocytes (inset). (G) Double mutants showed neoplastic regions of abnormal somatic cell proliferation in the posterior regions of the testes that were depleted of sperm. (G′) Enlargement of the somatic proliferation of double mutant testes. (H) Genetically wild-type testes depleted of germ cells due to dnd morpholino knockdown formed neoplasms of somatic cells of the testis similar to the ones observed in brca2;tp53 double mutants, but that invaded the intestine and muscle at older stages. (H′) Enlargement of the somatic proliferation of dnd testes. Abbreviations: asterisks (*), empty testis tubules; eo: early oocyte; i, intestine; m, muscle; ms, megalospermatogonia; nc, nucleolus; pc, pyknotic cell; sc, spermatocytes; sd, spermatids; sg, spermatogonia; sp, sperm; t, testis. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|