- Title
-
SNW1 Is a Critical Regulator of Spatial BMP Activity, Neural Plate Border Formation, and Neural Crest Specification in Vertebrate Embryos
- Authors
- Wu, M.Y., Ramel, M.C., Howell, M., and Hill, C.S.
- Source
- Full text @ PLoS Biol.
|
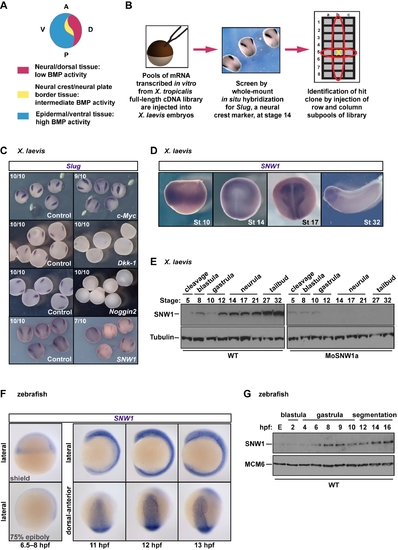
SNW1 was identified in a functional screen for neural crest fate in X. laevis. (A) A diagram of a stage 14 embryo depicting the classical model, which proposes that different levels of BMP signaling are required for ectodermal patterning and neural crest formation. (B) An overview of the functional screen. (C) The screen identified proteins whose overexpression modulated levels of Slug expression. 200 pg of mRNA expressing the X. tropicalis c-Myc, Dkk-1, Noggin2, or SNW1 was injected at the one-cell stage, with 250 pg of GFP mRNA as tracer. Control embryos received the GFP mRNA only. Embryos were fixed at stage 14 and analyzed for Slug expression by WISH. The number of embryos out of the total analyzed that showed the presented staining pattern is given. (D) X. laevis embryos were analyzed for SNW1 expression by WISH at the indicated stages. (E) Whole X. laevis embryo extracts were prepared from either wild-type (WT) embryos or embryos injected with 20 ng of a translation-blocking MO, MoSNW1a, at the one-cell stage, and analyzed by Western blotting using antibodies against SNW1 and Tubulin, the latter as a loading control. The developmental stages are indicated. (F) Zebrafish embryos were analyzed for SNW1 expression at the indicated times. Two gastrulation stages (the shield stage and 75% epiboly) and three segmentation stages (11, 12, and 13 hpf) are shown. Lateral views are shown, and for the later stages, also a dorsal-anterior view. (G) Whole zebrafish embryo extracts were prepared from wild-type embryos at the indicated times post-fertilization and analyzed by Western blotting using antibodies against SNW1 and MCM6, the latter as a loading control. The developmental stages are indicated. E, unfertilized egg. EXPRESSION / LABELING:
|
|
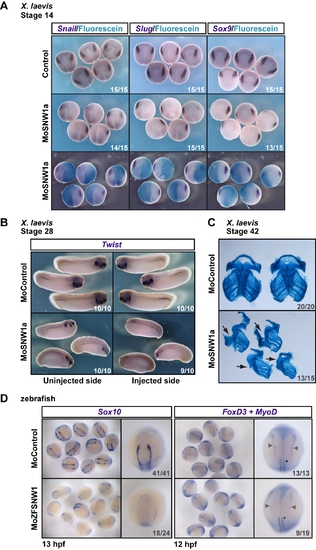
SNW1 knockdown inhibits neural crest induction. (A) Injection of MoSNW1a (Figure S3) results in the loss of neural crest markers Snail, Slug, and Sox9 on the injected side at stage 14. MoSNW1a (20 ng) was co-injected with Fdx as a tracer in one cell of a two-cell Xenopus embryo. The tracer was detected using an anti-fluorescein antibody. Control embryos were uninjected. (B) Two-cell Xenopus embryos were injected in one cell with either 20 ng of control MO (MoControl) or 20 ng of MoSNW1a. WISH was performed at stage 28 using a probe against Twist. (C) Xenopus embryos were injected with control MO or MoSNW1a as in (B). At stage 42 the facial cartilage was stained with alcian blue and then dissected. The injected side is indicated with an arrow. (D) Zebrafish embryos were injected with 15 ng of either a control MO or MoZFSNW1 and fixed at 13 hpf for WISH using a probe against the neural crest marker Sox10, or at 12 hpf for WISH with probes against FoxD3 and the adaxial and paraxial mesoderm marker MyoD. A group of embryos is shown, as well as a representative single embryo. The neural crest cells are marked by grey arrowheads, and the MyoD-postive cells by an asterisk (righthand panels). For the MyoD staining, only the adaxial mesoderm is visible, and this is not affected by SNW1 depletion. In all cases the number of embryos out of the total analyzed that showed the presented staining pattern or phenotype is given. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Dorsally expressed SNW1 is required for neural crest specification, and this is independent of mesoderm. (A) Left panels. Wild-type (WT) zebrafish embryos at the indicated times post-fertilization were doubly stained for the neural crest marker Sox10 (dark blue) and SNW1 (grey-purple), which is localized in the neural plate (see Figure 1F). Right panels. Wild-type stage 14 Xenopus embryos were stained for either SNW1, the neural crest marker Slug or the neural plate marker Sox3. The orange lines mark the edges of the neural crest staining to enable direct comparison of expression domains. (B) One dorsal or one ventral blastomere of four-cell Xenopus embryos was injected with Fdx with or without 10 ng of MoSNW1a, as indicated. At stage 14 the embryos were stained for Slug and fluorescein. (C) Xenopus embryos were either uninjected (control), injected with 20 ng of MoSNW1a at the one-cell stage, injected with 250 pg of CerS mRNA into all four blastomeres at the four-cell stage, or injected with MoSNW1a at the one-cell stage followed by CerS mRNA at the four-cell stage. WISH was carried out for the mesoderm markers Xbra and Gsc at stage 12, or the neural plate marker Sox3 and the neural crest marker Slug at stage 14. OE, overexpression. In all cases the number of embryos out of the total analyzed that showed the presented staining pattern is given. EXPRESSION / LABELING:
|
|
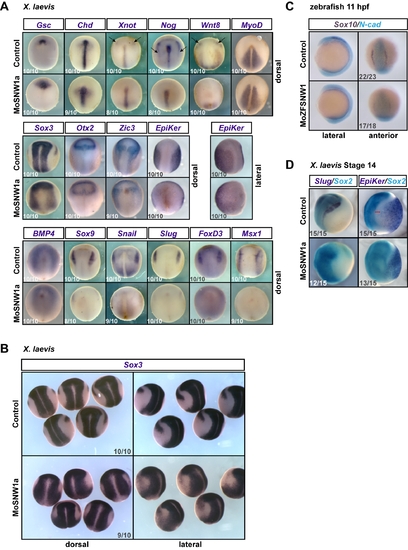
SNW1 is essential for specification of the border between neural and non-neural ectoderm. (A) Xenopus embryos were either uninjected (control) or injected with 20 ng of MoSNW1a at the one-cell stage. WISH was carried out for the dorsal mesoderm marker Goosecoid (Gsc), the dorsal midline markers Chordin (Chd), Xnot, and Noggin (Nog), the paraxial mesoderm markers Wnt8 and MyoD, the neural plate marker Sox3, the anterior neural marker Otx2, the anterior neural and neural plate border marker Zic3, the epidermal marker Epidermal keratin (EpiKer), and the neural plate border markers BMP4, Sox9, Snail, Slug, FoxD3, and Msx1. Arrows mark the expression of Xnot, Noggin, and Wnt8 at the anterior neural border. For EpiKer, a lateral view is shown in addition to the dorsal view. In all panels anterior is to the top. (B) Lateral and dorsal views of the Sox3 WISH are shown. Note that the sharp border of the neural plate is not formed when SNW1 is depleted. (C) Zebrafish embryos were injected with 15 ng of either a control MO (MoControl) or MoZFSNW1 and fixed at 11 hpf for double in situ hybridizations using probes against Sox10 (purple) and the neural plate marker N-cadherin (N-cad; turquoise). Lateral and anterior views of the same embryo are shown. (D) Xenopus embryos were either uninjected (control) or injected with 20 ng of MoSNW1a at the one-cell stage. Double in situ hybridizations were carried out at stage 14 for Slug (dark blue) and Sox2 (turquoise), or EpiKer (dark blue) and Sox2 (turquoise). The red line indicates the border between the neural plate and the epidermis. In all cases the number of embryos out of the total analyzed that showed the presented staining pattern is given. EXPRESSION / LABELING:
PHENOTYPE:
|
|
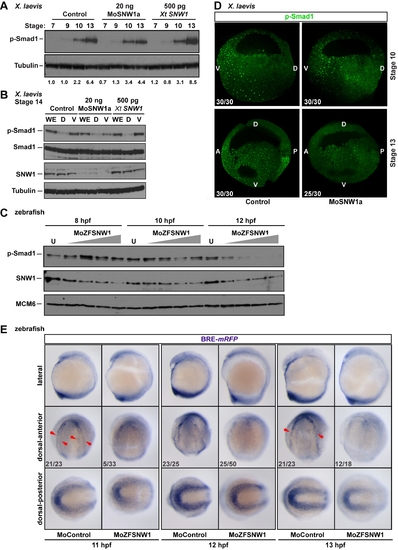
SNW1 regulates BMP activity in Xenopus and zebrafish embryos and is essential for BMP activity at the border between neural and non-neural tissue. (A) One-cell Xenopus embryos were either uninjected (control) or injected with 20 ng of MoSNW1a or 500 pg of X. tropicalis (Xt) SNW1. Embryos were harvested at the stages indicated, and whole embryo extracts were analyzed by Western blotting using antibodies against p-Smad1 and Tubulin, the latter as a loading control. Quantification of p-Smad1 levels relative to Tubulin is shown below the blots. (B) Xenopus embryos were either uninjected (control) or injected with 20 ng of MoSNW1a or 500 pg of X. tropicalis SNW1 mRNA at the one-cell stage, and bisected at stage 14 into dorsal (D) and ventral (V) halves. Whole cell extracts were analyzed by Western blotting using antibodies against SNW1, p-Smad1, Smad1, and Tubulin, the last as a loading control. WE, whole embryo. (C) Zebrafish embryos were either uninjected (U) or injected with increasing amounts (5, 10, 15, or 20 ng) of the splice-blocking MO MoZFSNW1. Embryos were harvested at 8, 10, or 12 hpf. Whole embryo extracts were analyzed by Western blotting using antibodies against SNW1, p-Smad1, and MCM6, the last as a loading control. (D) Knockdown of SNW1 strongly reduces ventral p-Smad1 levels at stage 13, but has no effect on the p-Smad1 ventral/dorsal gradient at stage 10. Embryos that were either uninjected (control) or injected with 20 ng of MoSNW1a at the one-cell stage were fixed at either stage 10 or stage 13, sagittally bisected along the midline, and immunostained with an antibody against p-Smad1. For these panels and subsequent p-Smad1 immunostaining, the specific p-Smad1 staining is nuclear and punctate. A, anterior; D, dorsal; P, posterior; V, ventral. (E) Transgenic BRE-mRFP embryos were injected with 15 ng of control MO or MoZFSNW1. They were fixed at 11, 12, and 13 hpf, when WISH was performed for mRFP, which indicates domains of BMP activity. In each case three different views of the same embryo are shown. In control embryos, mRFP is expressed in the tailbud (see dorsal-posterior view) and in a horseshoe-shaped domain at the dorsal anterior at 11 hpf (red arrowheads), which becomes increasingly sharpened at 12 and 13 hpf. In SNW1 morphants, the mRFP in this dorsal-anterior domain is reduced or absent. However, mRFP expression, and hence BMP activity, in the posterior is only slightly reduced or unchanged. In (D) and (E) the number of embryos out of the total analyzed that showed the presented staining pattern is given. In (E), the MO starts to knockdown SNW1 only from 8 hpf, and thus its effects become apparent only after 10 hpf. This explains why only a small percentage of embryos at 11 hpf shows the presented phenotype. By 13 hpf, when the MO effectively knocks down SNW1, 66% of the embryos show the phenotype. EXPRESSION / LABELING:
PHENOTYPE:
|
|
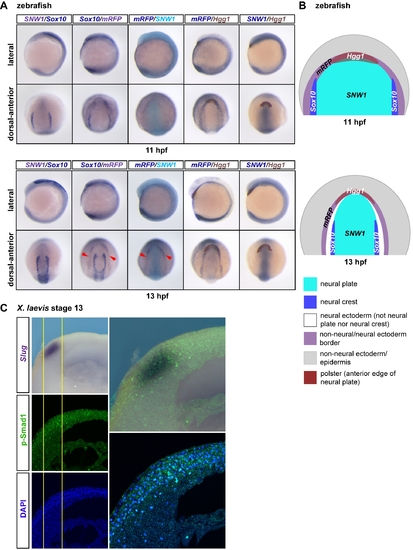
In post-gastrula zebrafish and Xenopus embryos the neural crest domain overlaps with the dorsal-anterior domain of BMP activity. (A) At early segmentation stages of zebrafish development the domains of expression of SNW1 and Sox10 partially overlap with the anterior “horseshoe” domain of BMP activity. Transgenic BRE-mRFP zebrafish embryos were stained for SNW1 (grey-purple) and Sox10 (dark blue), Sox10 (dark blue) and mRFP (grey-purple), mRFP (dark blue) and SNW1 (turquoise), mRFP (dark blue) and Hgg1 (maroon), or SNW1 (dark blue) and Hgg1 (maroon) at 11 hpf and 13 hpf. mRFP staining indicates regions of BMP activity. (B) A diagram representing the staining patterns of the four markers is shown. At 11 hpf, domains of expression of SNW1, Sox10, and mRFP partially overlap at the lateral edges of the dorsal-anterior horseshoe domain. However at 13 hpf, it is clear that although the neural crest marker Sox10 is still adjacent to the domain of SNW1 expression in the neural plate, the horseshoe mRFP expression domain (red arrowheads in [A]) is separated from the Sox10 and SNW1 expression domains. (C) Wild-type stage 13 Xenopus embryos were bisected transversely through the neural crest region. The anterior half was immunostained with an antibody against p-Smad1, and the posterior half was used for WISH against Slug. The embryos were then imaged along the plane of bisection and overlayed. The yellow lines outline the boundaries of detected p-Smad1 staining at the neural plate border, which overlaps with the Slug-positive neural crest cells. EXPRESSION / LABELING:
|
|
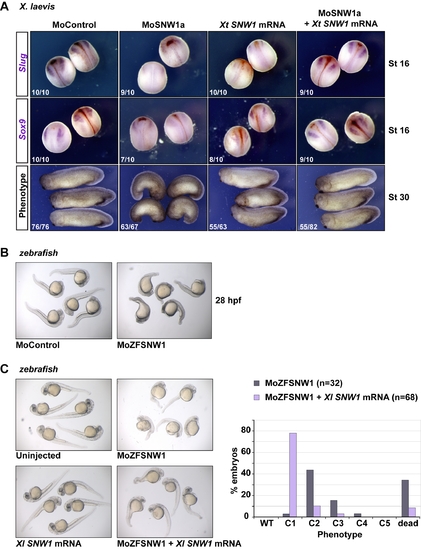
Overexpression of SNW1 can rescue the effects of SNW1 depletion in Xenopus and zebrafish. (A) The effects of SNW1 knockdown in X. laevis embryos can be rescued by overexpression of X. tropicalis SNW1. Embryos injected with 20 ng of control MO, 20 ng of MoSNW1a, 500 pg of X. tropicalis SNW1 mRNA, or both MoSNW1a and X. tropicalis SNW1 mRNA at the one-cell stage. Neural crest induction was assayed by WISH for Slug and Sox9 at stage 16. The phenotype was analyzed at stage 30. The number of embryos out of the total analyzed that showed the presented staining pattern/phenotype is given. (B) Zebrafish embryos were injected with 15 ng of either control MO or MoZFSNW1. They were photographed for phenotype analysis at 28 hpf. The morphant embryos present a dorsalized-like phenotype, but also display necrosis in the head, which is not rescued by p53 MO co-injection (data not shown [78]. (C) Overexpression of X. laevis SNW1 partially rescues the effects of SNW1 knockdown in zebrafish. Embryos were either uninjected or injected with 7.5 ng of MoZFSNW1, 125 pg of X. laevis SNW1 mRNA, or both. Embryos were cultured until 40 hpf, when they were analyzed for phenotype. MO-injected embryos and embryos injected with both the MO and the rescue mRNA were scored for a dorsalized phenotype (looking only at the extent of posterior structures) as in [71] (righthand graph). PHENOTYPE:
|
|
SNW1 knockdown has no effect on mesoderm induction or gastrulation and does not affect Activin-dependent induction of mesodermal tissue in Xenopus animal caps. (A) One-cell Xenopus embryos were injected with 20 ng of MoSNW1a. Uninjected control and MoSNW1a-injected embryos were fixed at stage 12 for WISH using probes against Xbra, Goosecoid, and Sizzled. (B) Animal caps dissected from MoSNW1a-injected embryos elongate similarly in response to 20 ng/ml Activin (PeproTech) as caps cut from control embryos. Thus mesoderm induction is not affected by injection of MoSNW1a. (C) Zebrafish embryos were injected with 15 ng of either control MO or MoZFSNW1. The embryos were fixed at 12 hpf and stained for the mesoderm markers No tail a (axial and tail), Even-skipped-like 1 (paraxial and tail), and Cdx4 (posterior axial and tail as well as some ectoderm). In SNW1 morphants, the expression of the mesoderm markers is largely unchanged, but there is a slight reduction in their expression in the tail mesoderm. Otx2 is a marker for anterior neural ectoderm and is normal albeit slightly expanded in SNW1 morphants. Otx2 and Cdx4 expression also indicate that anterior/posterior patterning is preserved in the absence of SNW1. In all cases the number of embryos out of the total analyzed that showed the presented staining pattern is given. |
|
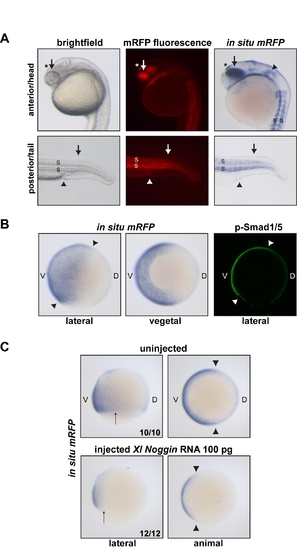
The BRE-mRFP transgenic zebrafish line is a reporter for BMP activity. A) 24-h transgenic embryos imaged using bright field or fluorescence microscopy, or processed for in situ hybridization using a probe against mRFP. mRFP protein or mRNA is indicative of BMP activity. Indeed, in the anterior/head region, mRFP is detected in known sites of BMP expression and/or activity such as the dorsal retina and lens (arrow; [79]) and the otic placode (arrowhead; [80]). mRFP is also strongly detected in the epiphysis/pineal gland, consistent with data obtained in Xenopus (asterisk; [81]). In the posterior/tail region, mRFP as a readout of BMP signaling is present for instance in the dorsal ectoderm, where BMP ligands are expressed (arrow; [82]), the cloaca (arrowhead; [83]), and the somites (S; [82]). (B) mRFP in situ hybridization on transgenic embryos at 85% epiboly. mRFP transcripts are detected in the ventral ectoderm and the ventral lateral plate and intermediate mesoderm, where BMP signaling is known to be active [26]. The mRFP pattern is consistent with p-Smad1/5 staining at the same stage (arrowheads; see also [4]). (C) Noggin overexpression inhibits mRFP transcription downstream of the BRE promoter. As expected, injection of X. laevis Noggin mRNA results in reduced BMP signaling [26], which leads to lower amounts of mRFP transcripts in 70% epiboly embryos. Arrows and arrowhead indicate the extent of the mRFP expression domain for comparison between uninjected and injected embryos. In (B) and (C), V, ventral; D, dorsal. The number of embryos out of the total analyzed that showed the presented staining pattern is given. EXPRESSION / LABELING:
|
|
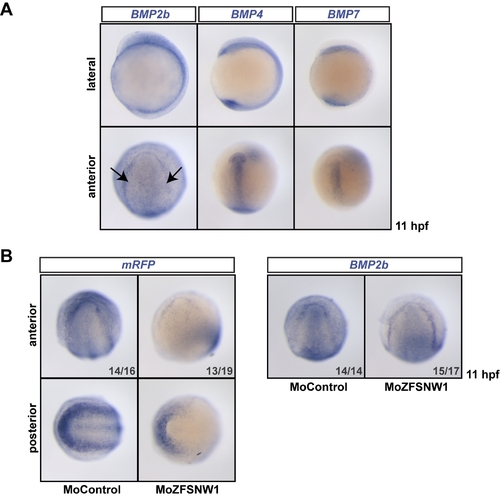
BMP2b is the BMP family member in zebrafish that accounts for the BMP activity at the epidermis/neural ectoderm border that is dependent on SNW1. (A) Comparison of BMP2b, BMP4, and BMP7 expression in zebrafish embryos at 11 hpf (2–3 somite stage) using WISH. BMP2b transcripts are enriched at the epidermis/neural ectoderm border (arrows). In contrast, BMP4 is enriched in the anterior prechordal plate and the tailbud, while BMP7 appears to be expressed in the endoderm. (B) Transgenic BRE-mRFP embryos were injected with 15 ng of control MO or MoZFSNW1. They were fixed at 11 hpf, when WISH was performed for mRFP. SNW1 knockdown results in the strong loss of BMP activity at the epidermis/neural ectoderm border, as seen with mRFP WISH in BRE-mRFP embryos. Notably, some of the posterior mRFP staining is preserved in the SNW1 morphant, which is likely because of BMP4 activity. BMP2b expression, however, appears unaltered in SNW1 morphants, suggesting that SNW1 does not induce BMP activity at the epidermis/neural ectoderm border through the transcriptional regulation of BMP2b. Note that the same batch of injected embryos was stained for mRFP or BMP2b. |