- Title
-
Restriction of hepatic competence by Fgf signaling
- Authors
- Shin, D., Lee, Y., Poss, K.D., and Stainier, D.Y.
- Source
- Full text @ Development
|
Wnt8a overexpression induces liver cells in the endoderm posterior to the liver. Embryos obtained from outcrossing Tg(hs:wnt8a) zebrafish were heat-shocked at 26 (A,B) or 22 (C-F) hpf, and processed for whole-mount immunostaining. (A,B) The Tg(fabp10:dsRed,ela3l:EGFP);Tg(ptf1a:eGFP);Tg(ins:dsRed) line was used to reveal hepatocytes (red), pancreatic acinar cells (green) and pancreatic β cells (red); mAb 2F11 staining reveals the hepatopancreatic duct (blue). The bracket marks the region with ectopic hepatocytes (n=7 out of 7). (C-F) Anti-Prox1 and anti-Pdx1 staining at 30 hpf (D; n=4 out of 4) reveals that Wnt8a overexpression induced Prox1 expression in endodermal cells located at the level of the second somite (S2) and blocked Pdx1 expression in these cells (the Pdx1 staining in the somites is due to non-reactivity as pdx1 is not expressed there). At 40 hpf, the endoderm posterior to the liver-forming region expressed Prox1 but not Pdx1 (F, bracket, n=4 out of 4). Dotted lines outline the dorsal pancreas, and S1-S3 represent the first, second and third somite, respectively. Ventral views, anterior up. Scale bar: 50 μm. |
|
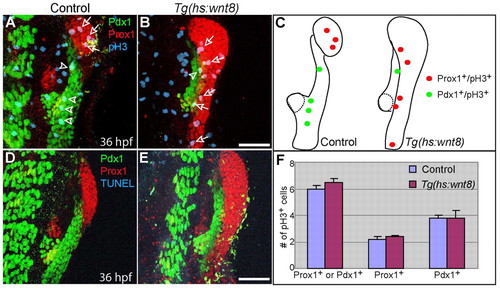
Wnt8a overexpression does not significantly affect cell proliferation or cell death in the endoderm 10 hours after heat-shock. Tg(hs:wnt8a) embryos were heat-shocked at 26 hpf and harvested at 36 hpf for whole-mount immunostaining. (A,B) Anti-Prox1 (red), anti-Pdx1 (green) and anti-pH3 (blue) triple labeling reveals that there was no significant difference in proliferation in Prox1+ or Pdx1+ endodermal cells between control (n=5 out of 5) and Wnt8a-overexpressing (n=4 out of 4) embryos 10 hours after heat-shock. Arrows point to Prox1+;pH3+ cells and arrowheads point to Pdx1+;pH3+ cells. (C) The distribution of pH3+ cells in A and B. Dotted lines outline the dorsal pancreas. (D,E) Anti-Prox1 (red), anti-Pdx1 (green) and TUNEL (blue) triple labeling reveals that no apoptosis was observed in Prox1+ or Pdx1+ endodermal cells of control or Wnt8-overexpressing embryos. (F) The number of pH3+ cells normalized over the size of the Prox1+ or Pdx1+ area are shown as a graph. Data are mean+s.e.m. Ventral views, anterior up. Scale bars: 50 μm. |
|
myca is specifically expressed in the liver-forming region, and is rapidly induced in the posterior endoderm upon Wnt8a overexpression. (A-D) myca starts to be expressed in the liver-forming region around 26 hpf (B, arrow) and is specifically expressed in the liver at least up to 48 hpf (C,D; arrows). (E-H) Wnt8a overexpression via heat-shock at 26 hpf induced ectopic myca expression in the posterior endoderm by 6 hours after heat-shock (F, bracket, n=10 out of 10) and ectopic expression can often be seen even 3 hours after heat-shock (H, n=13 out of 18). Dotted lines (G,H) delineate the endodermal region in which myca is expressed. Arrows in E,F point to the liver. Dorsal views, anterior to the left. Scale bars: 100 μm. |
|
Wnt8a overexpression induces the expression of various hepatic markers and represses the expression of an intestinal and a ventral pancreatic marker. Embryos obtained from outcrossing Tg(hs:wnt8a) zebrafish were heat-shocked at 26 hpf and harvested at 40 (I,J,M,N), 55 (A,B,K,L,O,P,S,T), 70 (Q,R) or 72 (C-H) hpf for in situ hybridization. (A-H) Wnt8a overexpression induced the expression of the hepatoblast marker hhex (B, n=10 out of 10), the hepatocyte markers cp (D, n=14 out of 14) and sepp1b (F, n=12 out of 14), and the hepatic ductal marker sox9b (H, n=13 out of 13) in the endoderm (brackets) posterior to the liver (arrows). Arrowheads indicate the dorsal pancreas (A,B). (I-P) By contrast, Wnt8a overexpression initially repressed the expression of the intestinal marker cdx1b (J, n=17 out of 17) and the early ventral pancreatic marker ptf1a (N, arrow, n=18 out of 18), but later their expression recovered (L,P), except for cdx1b expression in the intestinal bulb region (L, bracket, n=10 out of 10). Arrows in M-P point to the ventral pancreas or its progenitor cells. (Q-T) However, Wnt8a overexpression did not appear to affect the expression of other endodermal markers such as the swim bladder marker anxa5b (R, arrow, n=13 out of 13) or the thyroid marker nkx2.1a (T, arrow, n=10 out of 10). Arrows point to the swim bladder (Q,R) or thyroid (S,T). Dorsal (A-R) or side (S,T) views, anterior to the left. Scale bar: 100 μm. |
|
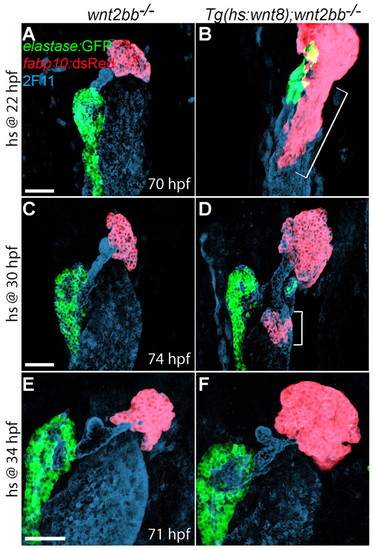
The extent of the endodermal region retaining hepatic competence is gradually reduced with time. (A-F) Embryos obtained from crossing wnt2bb-/- with Tg(hs:wnt8a);wnt2bb-/- zebrafish were heat-shocked at 22 (A,B), 30 (C,D) or 34 (E,F) hpf, and harvested at 70-74 hpf for whole-mount immunostaining. The Tg(fabp10:dsRed,ela3l:EGFP) line was used to reveal hepatocytes (red) and pancreatic acinar cells (green); mAb 2F11 staining reveals the hepatopancreatic duct (blue). Wnt8a overexpression at 22 hpf (B, bracket, n=6 out of 6) induced more ectopic hepatocytes than at 30 hpf (D, brackets, n=14 out of 14); Wnt8a overexpression at 34 hpf failed to induce ectopic hepatocytes (F, n=6 out of 6). However, the liver size in wnt2bb mutants increased even when Wnt8a was overexpressed at 34 hpf. Ventral views, anterior up. Scale bars: 50 μm. |
|
Fgf signaling negatively regulates hepatic competence. (A-H) Enhancing Fgf signaling by overexpressing caFgfr1 via heat-shock at 26 (A-D) or 30 (E-H) hpf greatly reduced or abolished the effect of Wnt8a overexpression on ectopic hepatocyte formation, whereas it did not block its effect on the increase of liver size in wnt2bb mutants. Fewer ectopic hepatocytes appeared to be induced in embryos that overexpressed both Wnt8a and caFgfr1 than in ones that overexpressed Wnt8a only (a representative comparison is shown in D versus C and H versus G, brackets, n=6 out of 6), but liver size was comparable between these embryos. (I-L) Reducing Fgf signaling with the Fgfr inhibitor SU5402 greatly enhanced the effect of Wnt8a overexpression on ectopic hepatocyte formation. Embryos that overexpressed Wnt8a from a 30 hpf heat-shock exhibited significantly more ectopic hepatocytes when treated with SU5402 from 24 to 38 hpf [L (n=6 out of 6) versus K (n=5 out of 5), brackets]. SU5402 treatment in the absence of Wnt8a overexpression did not induce ectopic hepatocytes, but increased liver size and reduced pancreatic size (J, n=5 out of 5). Ventral views, anterior up. Scale bars: 50 μm. |
|
fgf10a is expressed around the Pdx1+ domain. (A,D) fgf10a in situ hybridization combined with anti-GFP immunostaining in Tg(gutGFP) embryos reveals fgf10a expression around the endoderm directly caudal to the liver-forming region at 30 (A) and 36 (D) hpf. (B,E) Comparison with the Prox1 and Pdx1 expression pattern at these stages suggests that fgf10a is expressed around the Pdx1+ domain. (C,F) The expression patterns of Prox1 (red), Pdx1 (green) and fgf10a (blue) at these stages. Broken lines outline the dorsal pancreas; brackets mark the liver-forming region. Ventral views, anterior up. Scale bar: 50 μm. |
|
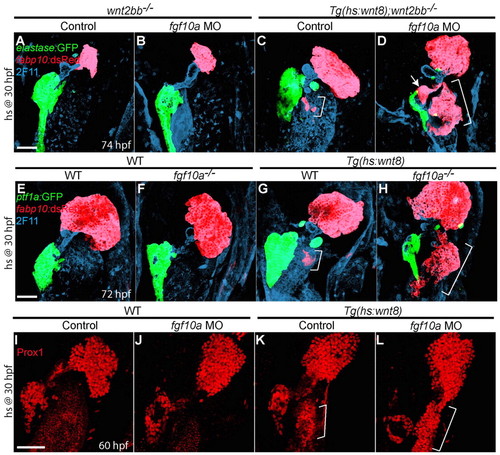
fgf10a knockdown greatly enhances ectopic hepatocyte formation induced by Wnt8a overexpression. (A-D,I-L) Embryos obtained from crossing wnt2bb-/- with Tg(hs:wnt8a);wnt2bb-/- zebrafish (A-D) or outcrossing Tg(hs:wnt8a) zebrafish (I-L) were injected with fgf10a MO, heat-shocked at 30 hpf, and harvested at 60 (I-L) or 74 (A-D) hpf for whole-mount immunostaining. (E-H) Embryos obtained from crossing fgf10a+/- with Tg(hs:wnt8a); fgf10a+/- zebrafish were heat-shocked at 30 hpf and harvested at 72 hpf for whole-mount immunostaining. fgf10a knockdown in wnt2bb mutants did not induce ectopic hepatocytes or affect liver size (B, n=8 out of 8), but it greatly enhanced ectopic hepatocyte formation induced by Wnt8a overexpression [D (n=6 out of 6) versus C (n=7 out of 7), brackets]. The ectopic hepatocytes were located in the intestinal bulb (brackets) and occasionally in the extrapancreatic duct (D, arrow). Ectopic hepatocyte formation was also greatly enhanced upon Wnt8a overexpression in fgf10a mutants (H, n=5 out of 5). Wnt8a overexpression greatly induced Prox1 expression in fgf10a MO-injected embryos compared with controls [L (n=5 out of 5) versus K (n=3 out of 3), brackets]. Ventral views, anterior up. Scale bars: 50 μm. |
|
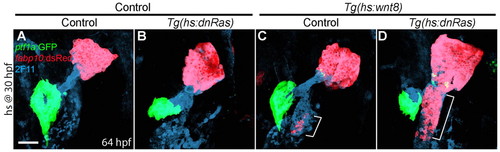
Blocking Ras function greatly enhances ectopic hepatocyte formation induced by Wnt8a overexpression. (A-D) Embryos obtained from crossing Tg(hs:wnt8a) with Tg(hs:dnRas) zebrafish were heat-shocked at 30 hpf, and harvested at 64 hpf for whole-mount immunostaining. Overexpression of dnRas did not induce ectopic hepatocytes or affect liver size in controls (B, n=5 out of 5), but it greatly enhanced ectopic hepatocyte formation induced by Wnt8a overexpression [D (n=7 out of 7) versus C (n=8 out of 8), brackets]. Ventral views, anterior upwards. Scale bar: 50 μm. |
|
Wnt/β-catenin signaling is essential for liver formation. (A-D) The Tg(fabp10:dsRed,ela3l:EGFP) line was used to reveal hepatocytes (red) and acinar pancreatic cells (green). The liver formed in most Tg(hs:dkk1) embryos that were heat-shocked at 18 hpf (B; n=21 out of 24) and in most wnt2bb mutants (C; n=460 out of 506), although liver size was smaller than wild type. However, the liver did not form in most Tg(hs:dkk1);wnt2bb-/- embryos heat-shocked at 18 hpf (D; 86%, n=50 out of 58). Arrows indicate the liver; brackets mark the extent of the pancreas. Dorsal views, anterior upwards. Scale bar: 100 μm. |
|
Bmp2b overexpression does not induce ectopic hepatocytes. (A-D) Embryos obtained from crossing wnt2bb-/- with Tg(hs:bmp2b);wnt2bb-/- zebrafish were heat-shocked at 22 (A,B) or 26 (C,D) hpf, and harvested at 73-75 hpf for whole-mount immunostaining. The Tg(fabp10:dsRed,ela3l:EGFP) line was used to reveal hepatocytes (red) and pancreatic acinar cells (green); mAb 2F11 staining reveals the hepatopancreatic duct (blue). Bmp2b overexpression did not induce ectopic hepatocytes but blocked the formation of the pancreatic tail (n=6 out of 6). Brackets mark the extent of the pancreatic tail. Ventral views, anterior upwards. Scale bar: 50 μm. |
|
Validation of the fgf10a splice MO. Embryos were injected with 4 ng of the fgf10a MO, which targets the boundary of the 1st exon and 1st intron, harvested at 28 hpf, and used for RT-PCR analysis. fgf10a cDNA including the 2nd exon and part of the 1st and 3rd exons was amplified from control but not fgf10a MO-injected embryos, indicating that the MO blocks the proper splicing of fgf10a transcript. Elongation factor 1a cDNA was amplified from both control and fgf10a MO-injected embryos. |
|
Expression patterns of foxa1/2/3 and gata4/6 in the endoderm. (A-E) Expression patterns of foxa1 (A), foxa2 (B), foxa3 (C), gata4 (D) and gata6 (E) at 26, 30, 38 and 48 hpf show that expression of these genes in the endoderm is fairly static at least during these stages. foxa1 expression is in the entire endoderm, whereas foxa2 and foxa3 expression are relatively restricted to the anterior and posterior region of the endoderm, respectively. Like foxa3, gata4 and gata6 expression appear restricted to the posterior region of the endoderm. Arrows indicate the liver. Dorsal views, anterior towards the left. Scale bar: 100 μm. |
|
Expression of foxa1/2/3 and gata4/6 appears unaffected in embryos that overexpress caFgfr1. Embryos obtained from outcrossing Tg(hsp70l:XlFgfr1) zebrafish were heat-shocked at 26 hpf, and harvested at 38 hpf for in situ hybridization. (A-J) The expression of foxa1 (A,B), foxa2 (C,D), foxa3 (E,F), gata4 (G,H) and gata6 (I,J), which encode factors implicated in hepatic competence, appears unaffected in embryos that overexpress caFgfr1 compared with controls (100%, n=10-12). Arrows indicate the liver. Dorsal views, anterior towards the left. Scale bar: 100 μm. |