- Title
-
Bisphenol A induces otolith malformations during vertebrate embryogenesis
- Authors
- Gibert, Y., Sassi-Messai, S., Fini, J.B., Bernard, L., Zalko, D., Cravedi, J.P., Balaguer, P., Andersson-Lendahl, M., Demeneix, B., and Laudet, V.
- Source
- Full text @ BMC Dev. Biol.
|
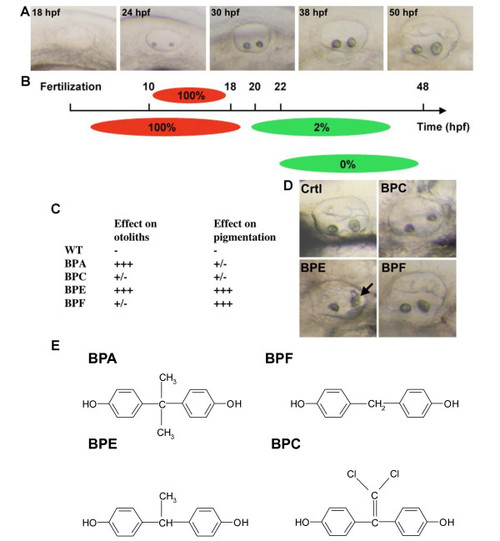
BPA treatment induces otolith abnormalities. A: BPA induced otolith malformation is dose dependent. Embryos were treated with different concentrations of BPA from 5 hpf onwards. Black bars represent embryos showing no otolith abnormalities, red bars represent treated embryos showing otolith abnormalities. (*: P < 0.01, Fisher test after Bonferroni correction). B: Anterior otolith (ao, upper left) and posterior otolith (po, upper middle) of control embryo and ao (lower left) and po (lower middle) of embryos treated with 70 µM BPA from 5 to 50 hpf showing otolith aggregation. In some rare cases a single otolith (upper right) or extra otolith (eo lower right) are observed. C: Upper panel: control embryo at 50 hpf (Upper right: close up of the otic vesicle). Lower panel: overall shape of BPA treated embryos form 6-50 hpf resembles control embryos, but display otolith aggregates (arrow). Lower right: close up of the otic vesicle in a BPA treated embryo form 24-50 hpf showing normal otoliths. D: After 75 hours of exposure, the concentration of BPA measured in embryos is 0.7 µg equivalent per mg fresh weight and 0.22 µg of BPF equivalent per mg fresh weight; see also Figure 2 C-E. E: BPA induces malformation of the otic vesicle in Xenopus embryos. Xenopus embryos were exposed to BPA (5 or 10 µM) from stage NF 18 to stage NF 40 (48 h) and examined at stage NF 45. The upper panel shows the morphology of the otoliths (or otoconia 73]) in control and BPA treated embryos. Note that 5 and 10 µM of BPA induce a progressive reduction in the size of the otoconia. The lower panel shows the semi-circular canals with a reduction in the distance between the two semi circular canals (arrowheads) induced by BPA. Similarly, the morphology of the developing semi circular canals is flattened under BPA treatment. |
|
Bisphenol effects are time and compound specific. A: Live pictures of the developing otic vesicle from 18 to 50 hpf in zebrafish embryo. B: BPA affects otolith in a restricted time window. Diagram showing the effect of 70 µM BPA treatments with different starting points or length of exposure. Upper panel: BPA pulse treatments (red bars) were performed from 10 hpf. At different stage of development (18, 24, 30 and 38 hpf) embryos were washed and developed in BPA free medium (gray bars). Otic vesicles were scored at 50 hpf. Lower panel: BPA acts prior 22 hpf to induce otolith defects. Treatments started prior or at 18 hpf lead to 100% of otolith defects. Treatment started from 20 hpf onwards lead to 85% of embryos with otolith defect. Treatment started at 22 hpf lead to only 2% of affected embryos. Treatments started later did not lead to any otolith defect. Red bars represent time when embryos were exposed to BPA. Gray bars represent time when embryos were not exposed to this compound. C: Various bisphenols affect otolith development and/or pigmentation. Embryos were treated from 5 to 72 hpf and malformed otolith or pigmentation defects scored (+++ indicates maximum defect, i.e. malformed otolith or total lack of pigment). Treatment of embryos with either BPA (70 µM) or BPE (70 µM) resulted in malformed otoliths. BPE also affected pigmentation, as did BPF (50 µM). BPC (70 µM) was without clear effects in these assays. D: Pictures of the effect of BPC, BPE and BPF on otolith development. All embryos were exposed to 70 µM of bisphenol. Note that only BPE gives an otolith phenotype similar to what in observed in BPA treated embryos with otolith aggregates marked by a black arrow. E: Chemical structures of BPA, BPC, BPE and BPF. |
|
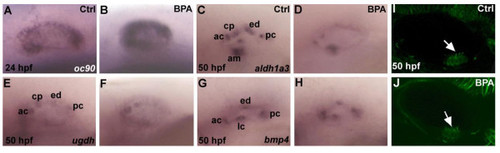
Expression of markers of inner ear development in zebrafish embryos are affected by BPA treatment. (A,B)oc90 a gene require for otolith formation in zebrafish is up-regulated under BPA treatment at 24 hpf (n=30). In situ for control and BPA treated embryos were performed in the same tube with the tip of the tail removed for the control embryos. (C,D)aldh1a3 is detected in the anterior cristae (ac), the cranial epithelial projection (cp), the endolymphatic duct (ed), the posterior cristae (pc) and the anterior macula (am) in wild type zebrafish embryo at 50 hpf (M), its expression is reduced in ac, cp and am and is absent in ed and pc in BPA treated embryos from 6 hpf onward (D). (E,F) Expression of ugdh at 50 hpf in control otic vesicle (E) is detected in the ac, cp, ed and pc. In BPA treated embryos from 6 hpf onwards (D), ugdh expression is severely reduced and remains solely detected in the ac and the cp. (G,H) Expression of bmp4 in control embryos at at 50 hpf (G) in the anterior (ac) lateral (lc) in the posterior cristae (pc) and the endolymphatic duct (ed) remains unaffected after BPA treatment (H). (I,J) Confocal microscopy of acetulated tubulin antibody staining showing the presence of the ciliated macula (white arrow) in control embryos (I) and in BPA treated embryos (white arrow in J). |