- Title
-
Disruption of planar cell polarity activity leads to developmental biliary defects
- Authors
- Cui, S., Capecci, L.M., and Matthews, R.P.
- Source
- Full text @ Dev. Biol.
|
Expression of PCP genes in 3 dpf zebrafish larvae. Whole-mount RNA in situ hybridization of PCP genes prickle 1a (pk1a) (A), dishevelled 2 (dsh2) (B), van gogh-like 2 (vangl2) (C), dishevelled 3 (dsh3) (D), wnt11 (E), wnt11r (F), ankyrin-related domain containing 6 (ankrd6) (G), and prickle 2 (pk2) (H). Note the liver staining (black arrows) present for A–G, but not in H. |
|
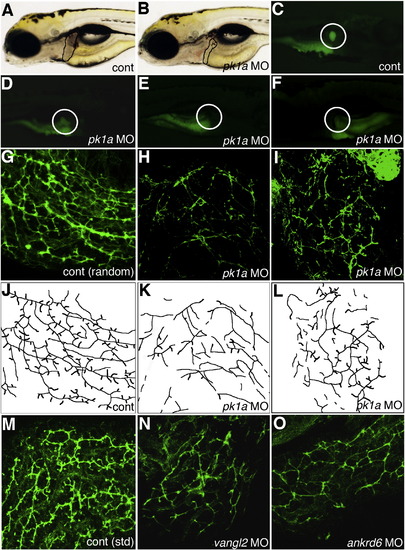
Altered PED6 gallbladder uptake and bile duct defects in pk1a morphants. (A–B) Left lateral view of live 5 dpf control (A, cont) and pk1a morpholino-injected (B, pk1a MO) larvae. Liver size (black outline) appears smaller in (B), but otherwise the larvae appear similar. (C–F) PED6 uptake in control (C) and three examples of pk1a morphants (D–F), showing decreased uptake (D), no uptake (E), and abnormal left-sided placement of the gallbladder (F) in pk1a morphants. Note that the view of (C–E) is right lateral and that of (F) is left lateral to show the gallbladder sidedness. (G–I) Confocal projections of whole-mount cytokeratin immunostainings of random control MO-injected (G) and pk1a morphant (H, I) livers at 5 dpf demonstrate decreased number and complexity of intrahepatic bile ducts in the pk1a morphants. (J–L) Line schematics of ducts in (G–I) to clarify the duct staining pattern. (M–O) Confocal projections of whole-mount cytokeratin immunostainings of livers from 5 dpf larvae injected with standard (std) control MO (M) and larvae injected with MOs against vangl2 (N) or ankrd6 (O). Note that the pattern of the ducts in (N) and (O) is similar to the ducts in the pk1a MO-injected larva (H, I). Similar results were obtained with either the AUG or splice blocking pk1a, vangl2, and ankrd6 MOs. PHENOTYPE:
|
|
Abnormal liver localization in pk1a morphants. Whole-mount in situ hybridization of ceruloplasmin (cp), a liver marker, in 3 dpf pk1a morphants demonstrates abnormal liver location, including “right extended” (B), “bilateral” (C), and “right” (D). (E) Graph depicting the scoring of pk1a morphants demonstrates a significant difference in the total number of abnormally localized livers (9% vs. 67%, p < 0.0001 by chi-square test), while the difference in livers limited to the right side is not significant (9% vs. 8%). Please see supplemental data for localization defects in other organs. PHENOTYPE:
|
|
Effect of pk1a on biliary development. (A, B) Bar graphs depicting PED6 uptake in control (cont), pk1a morpholino-injected (pk1a MO), pk1a mRNA-injected, and pk1a MO and mRNA-injected larvae. In (A), the percentage of larvae with no gallbladder (GB) uptake, faint uptake, and normal uptake is depicted on the Y-axis. In (B), numbers of larvae with abnormal left-sided GBs are depicted. Note that pk1a mRNA injection leads to an increase in the number of faint gallbladders compared to control (p < 0.0001), while mRNA injection leads to a rescue of the MO phenotype (p < 0.0001 compared to MO injection, NS compared to control). Forced expression of pk1a mRNA has no effect on GB sidedness, but does rescue the morphant sidedness phenotype. (C–E) Confocal projections of whole-mount cytokeratin staining of livers from 5 dpf control (B, cont), pk1a mRNA-injected (C) and pk1a MO and mRNA injection (D). Note that mRNA injection alone leads to scattered short duct-like structures, while mRNA and MO co-injection leads to rescue of the MO phenotype. |
|
Abnormal bile duct cells in pk1a morphant livers. (A–B) Single confocal optical slices of whole-mount 2F11 and PCNA immunostaining of livers from 5 dpf control (A, cont) and pk1a morphant (B) larvae. 2F11 staining is in green, PCNA in red, and DAPI counterstain in blue. Hepatocytes are noted with small white dots, while biliary cells are noted with larger dots. Solid dots represent cells that are PCNA positive, while the open dots are PCNA negative. Note that the biliary cells in control are all PCNA positive, while there are PCNA negative biliary cells in the pk1a morphant. (C-F) Electron micrographs from livers from control (C, E, cont) and pk1a morpholino-injected (D, F, pk1a MO) larvae. (C–D) Low power views (scale bar 2 μm) demonstrate overall similarity in appearance, but canaliculi (white arrowheads) appear to have accumulated material within in the morphants (D), and there is an accumulation of vesicles (black arrows) in the hepatocytes in (D). (E–F) Higher power views (scale bar 500 nm) demonstrate dilated Golgi (white arrow) in the bile duct cell in the morphant sample (F), as well as an accumulation of intracellular vesicles (F, white arrowhead). The white outlines circumscribe the bile duct cells in C–F. PHENOTYPE:
|
|
Inhibition of Rho kinase, JNK, and cytoskeletal architecture negatively affects biliary development. (A–F) Whole-mount projections of cytokeratin immunostaining of liver from a 5 dpf control larvae (A), compared to similar stainings from larvae treated with the Rho kinase inhibitors fasudil (B) and H-1152 (RKI, C), as well as the JNK inhibitor dicoumeral (D), the actin inhibitor cytochalasin D (E), and the microtubule and cytoskeleton inhibitor colchicine (F). Note that inhibition of any of the above downstream targets of PCP leads to a phenotype similar to the pk1a morphant phenotype. (G) Bar graph depicting PED6 treatment of control larvae compared to larvae treated with low dose pk1a morpholino, low dose cytochalasin D, or the combination. Note that alone, the low dose MO or cytochalasin have no to minimal effect, but that in combination, the effect is sizable and significant (p < 0.0005, chi-square). (H) Bar graph depicting PED6 treatment of control larvae compared to larvae treated with low dose pk1a morpholino, low dose colchicine, or the combination. Note that only in the combination is there a statistically significant effect on PED6 gallbladder uptake (p < 0.0001, chi-square test). (I–N) Confocal projections of cytokeratin immunostaining of livers from 5 dpf control larvae (I) and larvae injected with low dose (l.d.) pk1a MO (J), low dose cytochalasin D (cyto D, K), low dose colchicine (colch, L), and low dose pk1a MO with low dose cytochalasin D (M) or low dose colchicine (N). Note that (J–L) appear similar to control (I), but that (M, N) are abnormal. |
|
hnf6 and vhnf1 act downstream of PCP in zebrafish biliary development. (A) Quantitative real-time PCR of vhnf1 expression in control and pk1a MO-injected larvae demonstrating a 40% decrease in vhnf1 expression in the morphants. (B, C) In situ hybridizations of 3 dpf control (B, cont) and pk1a MO-injected larvae (C) showing expression of vhnf1 in pronephric ducts in both conditions (white arrowheads), but no expression in the morphant liver (white arrow) or intestine (i). (D) Epistasis experiment demonstrating that knockdown of pk1a and hnf6 is synergistic with respect to an effect on gallbladder PED6 intensity. (E) Confocal projection of cytokeratin immunostaining of livers from 5 dpf larvae injected with the combination of low dose pk1a and hnf6 MOs, showing a pattern of intrahepatic bile ducts similar to that seen after knockdown of pk1a, seen elsewhere. (F) Similar graph of PED6 gallbladder intensity for rescue of pk1a knockdown phenotype by vhnf1 mRNA injection demonstrating improvement in gallbladder intensity (p = 0.03). (G) Confocal projection of cytokeratin immunostaining of livers from 5 dpf larvae injected with pk1a MO with vhnf1 mRNA, demonstrating a pattern similar to control, seen elsewhere. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Documentation of morpholino knockdown. (A-D) PCR of 24 hpf embryos injected at the 1-cell stage with control and (A) ankrd6 morpholino (MO), (B) vangl2 MO, (C) pk1a MO, and each of the above (D), at the amounts used to elicit the phenotypes noted elsewhere (1.5 ng). (A) There is loss of a 375 bp ankrd6 PCR product spanning exon 4 and exon 6 (E4-E6) in embryos injected with an ankrd6 MO designed to inhibit splicing into exon 5. The lower part of the panel shows a 125 bp band using the same primers in the same embryos that results from loss of the ~250 bp exon 5. (B) A 202 bp vangl2 PCR product spanning exon 2 (E2) and the intron between exons 2 and 3 (I2) in embryos injected with a vangl2 MO designed to inhibit splicing out of I2, which introduces an in-frame stop codon from sequence within the second intron. (C) A novel 650 bp PCR product spanning pk1a exons 6 and 8 in embryos injected with pk1a MO designed to inhibit splicing into exon 7, as well as a decrease in a 200 bp PCR product spanning E6 and E7 in the same embryos. (D) Apparently similar amounts of a 100 bp tbp PCR product in embryos from each of the above knockdowns. The above results were confirmed using real-time quantitative PCR normalized against hprt or cp (ceruloplasmin). (E) Western blot analysis of 3 dpf larvae injected at 2 dpf with control (cont) or pk1a MOs. The upper panel shows a decrease in the 70 kDa Prickle band using anti-Prickle (αPk) antibody, while the lower shows constant Actin expression using αActin antibody. |
|
Abnormal intestine localization in pk1a morphants. Whole-mount in situ hybridization of fatty acid binding protein 2 (fabp2), an intestinal marker, in 3 dpf control (A) and pk1a morphants demonstrates abnormal intestine location, including “bilateral” (B), and “right” (C). (D) Graph depicting the scoring of pk1a morphants demonstrates a significant difference in the number of abnormally localized intestines (0% vs. 33%, p<0.0001 by chi-square test). Note that the fabp2 probe also faintly stains the liver. PHENOTYPE:
|
|
Marginally abnormal exocrine pancreas localization in pk1a morphants. Whole-mount in situ hybridization of trypsin (try), an exocrine pancreas marker, in 3 dpf control (A) and pk1a morphants demonstrates abnormal exocrine pancreas location, including “bilateral” (B), “midline” (C) and “left” (D). (E) Graph depicting the scoring of pk1a morphants demonstrates a slight difference in localization of the exocrine pancreas (p=NS by chi-square test). PHENOTYPE:
|
|
Abnormal endocrine pancreas localization in pk1a morphants. Wholemount in situ hybridization of insulin (ins), an endocrine pancreas marker, in 3 dpf control (A) and pk1a morphants demonstrates abnormal exocrine pancreas location, including “abnormal” (B, C), and “left” (D). (E) Graph depicting the scoring of pk1a morphants demonstrates a significant difference in the localization of the endocrine pancreas (p<0.0001 by chi-square test). When combined with the localization of try, there is a significant difference in the localization of the pancreas in pk1a morphants (p<0.0001). PHENOTYPE:
|
|
Additional examples of inhibition of Rho kinase, JNK, and cytoskeletal architecture negatively affecting biliary development. (A-F) Whole-mount projections of cytokeratin immunostaining of liver from a 5 dpf control larvae (A), compared to similar stainings from larvae treated with the Rho kinase inhibitors fasudil (B) and H- 1152 (RKI, C), as well as the JNK inhibitor dicoumeral (D), the actin inhibitor cytochalasin D (E), and the microtubule and cytoskeleton inhibitor colchicine (F). gb, gallbladder. |
|
Ceruloplasmin (cp) staining of 4 dpf larvae injected with pk1a MO. (A) In situ hybridization of 4 dpf larva injected with control (cont) MO demonstrating liver staining (white arrow). (B-C) In situ hybridizations of 4 dpf larvae injected with pk1a MO demonstrating liver staining (white arrow) similar to (A). Views in A and B are left lateral, while that in C is right lateral, showing the liver on the right side, similar to other studies depicted here. |

Unillustrated author statements EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 351(2), Cui, S., Capecci, L.M., and Matthews, R.P., Disruption of planar cell polarity activity leads to developmental biliary defects, 229-241, Copyright (2011) with permission from Elsevier. Full text @ Dev. Biol.